Genetic engineering of murine CD8+ and CD4+ T cells for preclinical adoptive immunotherapy studies
- PMID: 21499127
- PMCID: PMC3100770
- DOI: 10.1097/CJI.0b013e3182187600
Genetic engineering of murine CD8+ and CD4+ T cells for preclinical adoptive immunotherapy studies
Abstract
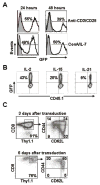
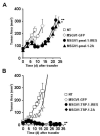
T-cell receptor (TCR) gene therapy enables for the rapid creation of antigen-specific T cells from mice of any strain and represents a valuable tool for preclinical immunotherapy studies. Here, we describe the superiority of γ-retroviral vectors compared with lentiviral vectors for transduction of murine T cells and surprisingly illustrate robust gene-transfer into phenotypically naive/memory-stem cell like (TN/TSCM; CD62L(hi)/CD44(low)) and central memory (TCM; CD62L(hi)/CD44(hi)) CD8+ T cells using murine stem cell-based γ-retroviral vectors (MSGV1). We created MSGV1 vectors for a major histocompatibility complex-class I-restricted TCR specific for the melanocyte-differentiation antigen, glycoprotein 100 (MSGV1-pmel-1), and a major histocompatibility complex-class II-restricted TCR specific for tyrosinase-related protein-1 (MSGV1-TRP-1), and found that robust gene expression required codon optimization of TCR sequences for the pmel-1 TCR. To test for functionality, we adoptively transferred TCR-engineered T cells into mice bearing B16 melanomas and observed delayed growth of established tumors with pmel-1 TCR engineered CD8+ T cells and significant tumor regression with TRP-1 TCR transduced CD4 T cells. We simultaneously created lentiviral vectors encoding the pmel-1 TCR, but found that these vectors mediated low TCR expression in murine T cells, but robust gene expression in other murine and human cell lines. These results indicate that preclinical murine models of adoptive immunotherapies are more practical using γ-retroviral rather than lentiviral vectors.
Conflict of interest statement
The authors declare that there are no potential conflicts of interests
Figures


Similar articles
-
T-cell receptor gene therapy of established tumors in a murine melanoma model.J Immunother. 2008 Jan;31(1):1-6. doi: 10.1097/CJI.0b013e31815c193f. J Immunother. 2008. PMID: 18157006 Free PMC article.
-
Both CD4 and CD8 T cells mediate equally effective in vivo tumor treatment when engineered with a highly avid TCR targeting tyrosinase.J Immunol. 2010 Jun 1;184(11):5988-98. doi: 10.4049/jimmunol.1000189. Epub 2010 Apr 28. J Immunol. 2010. PMID: 20427771 Free PMC article.
-
Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor.Cancer Res. 2005 Feb 15;65(4):1570-6. doi: 10.1158/0008-5472.CAN-04-2076. Cancer Res. 2005. PMID: 15735047
-
Molecular immunology lessons from therapeutic T-cell receptor gene transfer.Immunology. 2010 Feb;129(2):170-7. doi: 10.1111/j.1365-2567.2009.03227.x. Immunology. 2010. PMID: 20561357 Free PMC article. Review.
-
Needle in a Haystack: The Naïve Repertoire as a Source of T Cell Receptors for Adoptive Therapy with Engineered T Cells.Int J Mol Sci. 2020 Nov 6;21(21):8324. doi: 10.3390/ijms21218324. Int J Mol Sci. 2020. PMID: 33171940 Free PMC article. Review.
Cited by
-
Constitutive Lck Activity Drives Sensitivity Differences between CD8+ Memory T Cell Subsets.J Immunol. 2016 Jul 15;197(2):644-54. doi: 10.4049/jimmunol.1600178. Epub 2016 Jun 6. J Immunol. 2016. PMID: 27271569 Free PMC article.
-
Stem Cell-Derived Viral Antigen-Specific T Cells Suppress HBV Replication through Production of IFN-γ and TNF-⍺.iScience. 2020 Jul 24;23(7):101333. doi: 10.1016/j.isci.2020.101333. Epub 2020 Jul 1. iScience. 2020. PMID: 32679546 Free PMC article.
-
Cell-mediated delivery of nanoparticles: taking advantage of circulatory cells to target nanoparticles.J Control Release. 2014 Sep 28;190:531-41. doi: 10.1016/j.jconrel.2014.03.050. Epub 2014 Apr 18. J Control Release. 2014. PMID: 24747161 Free PMC article. Review.
-
Expansion and Retroviral Transduction of Primary Murine T Cells for CAR T-Cell Therapy.Methods Mol Biol. 2024;2748:41-53. doi: 10.1007/978-1-0716-3593-3_4. Methods Mol Biol. 2024. PMID: 38070106
-
Molecular features of the complementarity determining region 3 motif of the T cell population and subsets in the blood of patients with chronic severe hepatitis B.J Transl Med. 2011 Dec 8;9:210. doi: 10.1186/1479-5876-9-210. J Transl Med. 2011. PMID: 22152113 Free PMC article.
References
-
- Rosenberg SA. Immunotherapy and gene therapy of cancer. Cancer Res. 1991;51:5074s–9s. - PubMed
-
- Hwu P, Rosenberg SA. The genetic modification of T cells for cancer therapy: an overview of laboratory and clinical trials. Cancer Detect Prev. 1994;18:43–50. - PubMed
-
- Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

