IQGAP1 regulates ERK1/2 and AKT signalling in the heart and sustains functional remodelling upon pressure overload
- PMID: 21493702
- PMCID: PMC3294280
- DOI: 10.1093/cvr/cvr103
IQGAP1 regulates ERK1/2 and AKT signalling in the heart and sustains functional remodelling upon pressure overload
Abstract
Aims: The Raf-MEK1/2-ERK1/2 (ERK1/2-extracellular signal-regulated kinases 1/2) signalling cascade is crucial in triggering cardiac responses to different stress stimuli. Scaffold proteins are key elements in coordinating signalling molecules for their appropriate spatiotemporal activation. Here, we investigated the role of IQ motif-containing GTPase-activating protein 1 (IQGAP1), a scaffold for the ERK1/2 cascade, in heart function and remodelling in response to pressure overload.
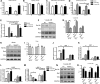
Methods and results: IQGAP1-null mice have unaltered basal heart function. When subjected to pressure overload, IQGAP1-null mice initially develop a compensatory hypertrophy indistinguishable from that of wild-type (WT) mice. However, upon a prolonged stimulus, the hypertrophic response develops towards a thinning of left ventricular walls, chamber dilation, and a decrease in contractility, in an accelerated fashion compared with WT mice. This unfavourable cardiac remodelling is characterized by blunted reactivation of the foetal gene programme, impaired cardiomyocyte hypertrophy, and increased cardiomyocyte apoptosis. Analysis of signalling pathways revealed two temporally distinct waves of both ERK1/2 and AKT phosphorylation peaking, respectively, at 10 min and 4 days after aortic banding in WT hearts. IQGAP1-null mice show strongly impaired phosphorylation of MEK1/2-ERK1/2 and AKT following 4 days of pressure overload, but normal activation of these kinases after 10 min. Pull-down experiments indicated that IQGAP1 is able to bind the three components of the ERK cascade, namely c-Raf, MEK1/2, and ERK1/2, as well as AKT in the heart.
Conclusion: These data demonstrate, for the first time, a key role for the scaffold protein IQGAP1 in integrating hypertrophy and survival signals in the heart and regulating long-term left ventricle remodelling upon pressure overload.
Figures
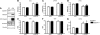
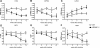


Similar articles
-
Assessing the role of extracellular signal-regulated kinases 1 and 2 in volume overload-induced cardiac remodelling.ESC Heart Fail. 2019 Oct;6(5):1015-1026. doi: 10.1002/ehf2.12497. Epub 2019 Jul 19. ESC Heart Fail. 2019. PMID: 31322843 Free PMC article.
-
ERK1/2 activation in heart is controlled by melusin, focal adhesion kinase and the scaffold protein IQGAP1.J Cell Sci. 2011 Oct 15;124(Pt 20):3515-24. doi: 10.1242/jcs.091140. J Cell Sci. 2011. PMID: 22010199 Free PMC article.
-
Baicalein attenuates angiotensin II-induced cardiac remodeling via inhibition of AKT/mTOR, ERK1/2, NF-κB, and calcineurin signaling pathways in mice.Am J Hypertens. 2015 Apr;28(4):518-26. doi: 10.1093/ajh/hpu194. Epub 2014 Oct 31. Am J Hypertens. 2015. PMID: 25362112
-
Intrinsic and acquired resistance to MEK1/2 inhibitors in cancer.Biochem Soc Trans. 2014 Aug;42(4):776-83. doi: 10.1042/BST20140129. Biochem Soc Trans. 2014. PMID: 25109957 Review.
-
IQGAP1 and its binding proteins control diverse biological functions.Cell Signal. 2012 Apr;24(4):826-34. doi: 10.1016/j.cellsig.2011.12.005. Epub 2011 Dec 11. Cell Signal. 2012. PMID: 22182509 Free PMC article. Review.
Cited by
-
Melusin Promotes a Protective Signal Transduction Cascade in Stressed Hearts.Front Mol Biosci. 2016 Sep 12;3:53. doi: 10.3389/fmolb.2016.00053. eCollection 2016. Front Mol Biosci. 2016. PMID: 27672636 Free PMC article. Review.
-
An intrinsic mechanism of metabolic tuning promotes cardiac resilience to stress.EMBO Mol Med. 2024 Oct;16(10):2450-2484. doi: 10.1038/s44321-024-00132-z. Epub 2024 Sep 13. EMBO Mol Med. 2024. PMID: 39271959 Free PMC article.
-
IQ Domain-Containing GTPase-Activating Protein 1 Regulates Cytoskeletal Reorganization and Facilitates NKG2D-Mediated Mechanistic Target of Rapamycin Complex 1 Activation and Cytokine Gene Translation in Natural Killer Cells.Front Immunol. 2018 May 28;9:1168. doi: 10.3389/fimmu.2018.01168. eCollection 2018. Front Immunol. 2018. PMID: 29892299 Free PMC article.
-
Surface functionalization of polyurethane scaffolds mimicking the myocardial microenvironment to support cardiac primitive cells.PLoS One. 2018 Jul 6;13(7):e0199896. doi: 10.1371/journal.pone.0199896. eCollection 2018. PLoS One. 2018. PMID: 29979710 Free PMC article.
-
IQGAP1: insights into the function of a molecular puppeteer.Mol Immunol. 2015 Jun;65(2):336-49. doi: 10.1016/j.molimm.2015.02.012. Epub 2015 Feb 28. Mol Immunol. 2015. PMID: 25733387 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

