Naturally occurring autoantibodies against beta-amyloid: investigating their role in transgenic animal and in vitro models of Alzheimer's disease
- PMID: 21490226
- PMCID: PMC6622820
- DOI: 10.1523/JNEUROSCI.4401-10.2011
Naturally occurring autoantibodies against beta-amyloid: investigating their role in transgenic animal and in vitro models of Alzheimer's disease
Abstract
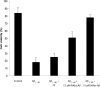
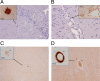
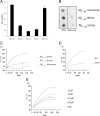
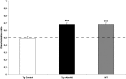
Alzheimer's disease (AD) is a neurodegenerative disorder primarily affecting regions of the brain responsible for higher cognitive functions. Immunization against β-amyloid (Aβ) in animal models of AD has been shown to be effective on the molecular level but also on the behavioral level. Recently, we reported naturally occurring autoantibodies against Aβ (NAbs-Aβ) being reduced in Alzheimer's disease patients. Here, we further investigated their physiological role: in epitope mapping studies, NAbs-Aβ recognized the mid-/C-terminal end of Aβ and preferentially bound to oligomers but failed to bind to monomers/fibrils. NAbs-Aβ were able to interfere with Aβ peptide toxicity, but NAbs-Aβ did not readily clear senile plaques although early fleecy-like plaques were reduced. Administration of NAbs-Aβ in transgenic mice improved the object location memory significantly, almost reaching performance levels of wild-type control mice. These findings suggest a novel physiological mechanism involving NAbs-Aβ to dispose of proteins or peptides that are prone to forming toxic aggregates.
Figures

Similar articles
-
Naturally occurring autoantibodies against Aβ oligomers exhibited more beneficial effects in the treatment of mouse model of Alzheimer's disease than intravenous immunoglobulin.Neuropharmacology. 2016 Jun;105:561-576. doi: 10.1016/j.neuropharm.2016.02.015. Epub 2016 Feb 18. Neuropharmacology. 2016. PMID: 26907803
-
Naturally occurring autoantibodies against β-Amyloid.Adv Exp Med Biol. 2012;750:91-9. doi: 10.1007/978-1-4614-3461-0_7. Adv Exp Med Biol. 2012. PMID: 22903668 Review.
-
Nonhuman amyloid oligomer epitope reduces Alzheimer's-like neuropathology in 3xTg-AD transgenic mice.Mol Neurobiol. 2013 Dec;48(3):931-40. doi: 10.1007/s12035-013-8478-7. Epub 2013 Jun 15. Mol Neurobiol. 2013. PMID: 23771815
-
Tau passive immunization inhibits not only tau but also Aβ pathology.Alzheimers Res Ther. 2017 Jan 10;9(1):1. doi: 10.1186/s13195-016-0227-5. Alzheimers Res Ther. 2017. PMID: 28073379 Free PMC article.
-
Developing novel immunogens for a safe and effective Alzheimer's disease vaccine.Prog Brain Res. 2009;175:83-93. doi: 10.1016/S0079-6123(09)17506-4. Prog Brain Res. 2009. PMID: 19660650 Free PMC article. Review.
Cited by
-
Immunotherapy in prion disease.Nat Rev Neurol. 2013 Feb;9(2):98-105. doi: 10.1038/nrneurol.2012.258. Epub 2012 Dec 18. Nat Rev Neurol. 2013. PMID: 23247613 Review.
-
Tau-targeted treatment strategies in Alzheimer's disease.Br J Pharmacol. 2012 Mar;165(5):1246-59. doi: 10.1111/j.1476-5381.2011.01713.x. Br J Pharmacol. 2012. PMID: 22044248 Free PMC article. Review.
-
Safflower yellow attenuates learning and memory deficits in amyloid β-induced Alzheimer's disease rats by inhibiting neuroglia cell activation and inflammatory signaling pathways.Metab Brain Dis. 2019 Jun;34(3):927-939. doi: 10.1007/s11011-019-00398-0. Epub 2019 Mar 4. Metab Brain Dis. 2019. PMID: 30830599
-
Increased levels of antigen-bound β-amyloid autoantibodies in serum and cerebrospinal fluid of Alzheimer's disease patients.PLoS One. 2013 Jul 18;8(7):e68996. doi: 10.1371/journal.pone.0068996. Print 2013. PLoS One. 2013. PMID: 23874844 Free PMC article.
-
Intravenous immunglobulin binds beta amyloid and modifies its aggregation, neurotoxicity and microglial phagocytosis in vitro.PLoS One. 2013 May 16;8(5):e63162. doi: 10.1371/journal.pone.0063162. Print 2013. PLoS One. 2013. PMID: 23696796 Free PMC article.
References
-
- Bacher M, Dodel R, Aljabari B, Keyvani K, Marambaud P, Kayed R, Glabe C, Goertz N, Hoppmann A, Sachser N, Klotsche J, Schnell S, Lewejohann L, Al-Abed Y. CNI-1493 inhibits Abeta production, plaque formation, and cognitive deterioration in an animal model of Alzheimer's disease. J Exp Med. 2008;205:1593–1599. - PMC - PubMed
-
- Bacher M, Depboylu C, Du Y, Noelker C, Oertel WH, Behr T, Henriksen G, Behe M, Dodel R. Peripheral and central biodistribution of (111)In-labeled anti-beta-amyloid autoantibodies in a transgenic mouse model of Alzheimer's disease. Neurosci Lett. 2009;449:240–245. - PubMed
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
-
- Britschgi M, Olin CE, Johns HT, Takeda-Uchimura Y, LeMieux MC, Rufibach K, Rajadas J, Zhang H, Tomooka B, Robinson WH, Clark CM, Fagan AM, Galasko DR, Holtzman DM, Jutel M, Kaye JA, Lemere CA, Leszek J, Li G, Peskind ER, Quinn JF, Yesavage JA, Ghiso JA, Wyss-Coray T. Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:12145–12150. - PMC - PubMed
-
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
