Reduced BACE1 activity enhances clearance of myelin debris and regeneration of axons in the injured peripheral nervous system
- PMID: 21490216
- PMCID: PMC3302726
- DOI: 10.1523/JNEUROSCI.6810-10.2011
Reduced BACE1 activity enhances clearance of myelin debris and regeneration of axons in the injured peripheral nervous system
Abstract
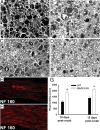
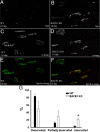
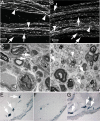
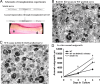
β-Site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1) is an aspartyl protease best known for its role in generating the amyloid-β peptides that are present in plaques of Alzheimer's disease. BACE1 has been an attractive target for drug development. In cultured embryonic neurons, BACE1-cleaved N-terminal APP is further processed to generate a fragment that can trigger axonal degeneration, suggesting a vital role for BACE1 in axonal health. In addition, BACE1 cleaves neuregulin 1 type III, a protein critical for myelination of peripheral axons by Schwann cells during development. Here, we asked whether axonal degeneration or axonal regeneration in adult nerves might be affected by inhibition or elimination of BACE1. We report that BACE1 knock-out and wild-type nerves degenerated at a similar rate after axotomy and to a similar extent in the experimental neuropathies produced by administration of paclitaxel and acrylamide. These data indicate N-APP is not the sole culprit in axonal degeneration in adult nerves. Unexpectedly, however, we observed that BACE1 knock-out mice had markedly enhanced clearance of axonal and myelin debris from degenerated fibers, accelerated axonal regeneration, and earlier reinnervation of neuromuscular junctions, compared with littermate controls. These observations were reproduced in part by pharmacological inhibition of BACE1. These data suggest BACE1 inhibition as a therapeutic approach to accelerate regeneration and recovery after peripheral nerve damage.
Figures


Similar articles
-
Axonal and Schwann cell BACE1 is equally required for remyelination of peripheral nerves.J Neurosci. 2015 Mar 4;35(9):3806-14. doi: 10.1523/JNEUROSCI.5207-14.2015. J Neurosci. 2015. PMID: 25740511 Free PMC article.
-
Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves.FASEB J. 2008 Aug;22(8):2970-80. doi: 10.1096/fj.08-106666. Epub 2008 Apr 15. FASEB J. 2008. PMID: 18413858 Free PMC article.
-
Increased BACE1 activity inhibits peripheral nerve regeneration after injury.Neurobiol Dis. 2017 Oct;106:147-157. doi: 10.1016/j.nbd.2017.07.003. Epub 2017 Jul 5. Neurobiol Dis. 2017. PMID: 28687442
-
BACE1 influences debris clearance and axonal regeneration in injured peripheral nerve.J Peripher Nerv Syst. 2012 Dec;17 Suppl 3:30-3. doi: 10.1111/j.1529-8027.2012.00428.x. J Peripher Nerv Syst. 2012. PMID: 23279429 Review.
-
The influence of BACE1 on macrophage recruitment and activity in the injured peripheral nerve.J Neuroinflammation. 2021 Mar 15;18(1):71. doi: 10.1186/s12974-021-02121-2. J Neuroinflammation. 2021. PMID: 33722254 Free PMC article. Review.
Cited by
-
Changes in the Foxj1 expression of Schwann cells after sciatic nerve crush.J Mol Histol. 2013 Aug;44(4):391-9. doi: 10.1007/s10735-013-9500-0. Epub 2013 Mar 21. J Mol Histol. 2013. PMID: 23515839
-
GCPII Inhibition Promotes Remyelination after Peripheral Nerve Injury in Aged Mice.Int J Mol Sci. 2024 Jun 23;25(13):6893. doi: 10.3390/ijms25136893. Int J Mol Sci. 2024. PMID: 39000003 Free PMC article.
-
Animal models of Alzheimer disease.Cold Spring Harb Perspect Med. 2012 Nov 1;2(11):a006320. doi: 10.1101/cshperspect.a006320. Cold Spring Harb Perspect Med. 2012. PMID: 23002015 Free PMC article. Review.
-
The Complex Work of Proteases and Secretases in Wallerian Degeneration: Beyond Neuregulin-1.Front Cell Neurosci. 2019 Mar 20;13:93. doi: 10.3389/fncel.2019.00093. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30949030 Free PMC article. Review.
-
Physiological Roles of Monomeric Amyloid-β and Implications for Alzheimer's Disease Therapeutics.Exp Neurobiol. 2022 Apr 30;31(2):65-88. doi: 10.5607/en22004. Exp Neurobiol. 2022. PMID: 35673997 Free PMC article. Review.
References
-
- Araki W, Kitaguchi N, Tokushima Y, Ishii K, Aratake H, Shimohama S, Nakamura S, Kimura J. Trophic effect of β-amyloid precursor protein on cerebral cortical neurons in culture. Biochem Biophys Res Commun. 1991;181:265–271. - PubMed
-
- Beirowski B, Berek L, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP. Quantitative and qualitative analysis of Wallerian degeneration using restricted axonal labelling in YFP-H mice. J Neurosci Methods. 2004;134:23–35. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
