Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells
- PMID: 21488066
- PMCID: PMC4158408
- DOI: 10.1002/hep.24341
Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells
Abstract
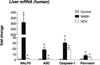
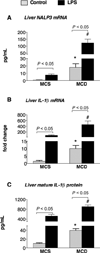
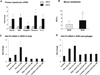
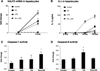
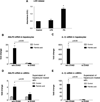
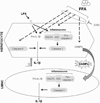
The pathogenesis of nonalcoholic steatohepatitis (NASH) and inflammasome activation involves sequential hits. The inflammasome, which cleaves pro-interleukin-1β (pro-IL-1β) into secreted IL-1β, is induced by endogenous and exogenous danger signals. Lipopolysaccharide (LPS), a toll-like receptor 4 ligand, plays a role in NASH and also activates the inflammasome. In this study, we hypothesized that the inflammasome is activated in NASH by multiple hits involving endogenous and exogenous danger signals. Using mouse models of methionine choline-deficient (MCD) diet-induced NASH and high-fat diet-induced NASH, we found up-regulation of the inflammasome [including NACHT, LRR, and PYD domains-containing protein 3 (NALP3; cryopyrin), apoptosis-associated speck-like CARD-domain containing protein, pannexin-1, and pro-caspase-1] at the messenger RNA (mRNA) level increased caspase-1 activity, and mature IL-1β protein levels in mice with steatohepatitis in comparison with control livers. There was no inflammasome activation in mice with only steatosis. The MCD diet sensitized mice to LPS-induced increases in NALP3, pannexin-1, IL-1β mRNA, and mature IL-1β protein levels in the liver. We demonstrate for the first time that inflammasome activation occurs in isolated hepatocytes in steatohepatitis. Our novel data show that the saturated fatty acid (FA) palmitic acid (PA) activates the inflammasome and induces sensitization to LPS-induced IL-1β release in hepatocytes. Furthermore, PA triggers the release of danger signals from hepatocytes in a caspase-dependent manner. These hepatocyte-derived danger signals, in turn, activate inflammasome, IL-1β, and tumor necrosis factor α release in liver mononuclear cells.
Conclusion: Our novel findings indicate that saturated FAs represent an endogenous danger in the form of a first hit, up-regulate the inflammasome in NASH, and induce sensitization to a second hit with LPS for IL-β release in hepatocytes. Furthermore, hepatocytes exposed to saturated FAs release danger signals that trigger inflammasome activation in immune cells. Thus, hepatocytes play a key role in orchestrating tissue responses to danger signals in NASH.
Copyright © 2011 American Association for the Study of Liver Diseases.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures

Similar articles
-
Dietary saturated fatty acid and polyunsaturated fatty acid oppositely affect hepatic NOD-like receptor protein 3 inflammasome through regulating nuclear factor-kappa B activation.World J Gastroenterol. 2016 Feb 28;22(8):2533-44. doi: 10.3748/wjg.v22.i8.2533. World J Gastroenterol. 2016. PMID: 26937141 Free PMC article.
-
Both bone marrow-derived and non-bone marrow-derived cells contribute to AIM2 and NLRP3 inflammasome activation in a MyD88-dependent manner in dietary steatohepatitis.Liver Int. 2014 Oct;34(9):1402-13. doi: 10.1111/liv.12537. Epub 2014 Apr 17. Liver Int. 2014. PMID: 24650018 Free PMC article.
-
Cathepsin B inhibition ameliorates the non-alcoholic steatohepatitis through suppressing caspase-1 activation.J Physiol Biochem. 2018 Nov;74(4):503-510. doi: 10.1007/s13105-018-0644-y. Epub 2018 Jul 17. J Physiol Biochem. 2018. PMID: 30019185
-
Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE).Brain Pathol. 2017 Mar;27(2):213-219. doi: 10.1111/bpa.12477. Brain Pathol. 2017. PMID: 27997058 Free PMC article. Review.
-
Inflammasome activation in the liver: Focus on alcoholic and non-alcoholic steatohepatitis.Clin Res Hepatol Gastroenterol. 2015 Sep;39 Suppl 1:S18-23. doi: 10.1016/j.clinre.2015.06.012. Epub 2015 Jul 26. Clin Res Hepatol Gastroenterol. 2015. PMID: 26216030 Review.
Cited by
-
Non-alcoholic Fatty Liver Disease and Alcohol-Related Liver Disease: Two Intertwined Entities.Front Med (Lausanne). 2020 Aug 20;7:448. doi: 10.3389/fmed.2020.00448. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32974366 Free PMC article. Review.
-
Dietary inflammatory index and the risks of non-alcoholic fatty liver disease: a systematic review and meta-analysis.Front Nutr. 2024 Jul 25;11:1388557. doi: 10.3389/fnut.2024.1388557. eCollection 2024. Front Nutr. 2024. PMID: 39119468 Free PMC article.
-
Murine Gammaherpesvirus 68 Pathogenesis Is Independent of Caspase-1 and Caspase-11 in Mice and Impairs Interleukin-1β Production upon Extrinsic Stimulation in Culture.J Virol. 2015 Jul;89(13):6562-74. doi: 10.1128/JVI.00658-15. Epub 2015 Apr 8. J Virol. 2015. PMID: 25855746 Free PMC article.
-
Inflammasome-Mediated Cytokines: A Key Connection between Obesity-Associated NASH and Liver Cancer Progression.Biomedicines. 2022 Sep 21;10(10):2344. doi: 10.3390/biomedicines10102344. Biomedicines. 2022. PMID: 36289606 Free PMC article. Review.
-
Caspase-1 deficiency in mice reduces intestinal triglyceride absorption and hepatic triglyceride secretion.J Lipid Res. 2013 Feb;54(2):448-56. doi: 10.1194/jlr.M031963. Epub 2012 Nov 17. J Lipid Res. 2013. PMID: 23160218 Free PMC article.
References
-
- Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. - PubMed
-
- Day CP, James OF. Steatohepatitis: a tale of two ‘hits’? Gastroenterology. 1998;114:842–845. - PubMed
-
- deAlmeida IT, Cortez-Pinto H, Fidalgo G, Rodrigues D, Camilo ME. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr. 2002;21:219–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
