Spatial-temporal modulation of CCN proteins during wound healing in human skin in vivo
- PMID: 21484592
- PMCID: PMC3058195
- DOI: 10.1007/s12079-010-0114-y
Spatial-temporal modulation of CCN proteins during wound healing in human skin in vivo
Abstract
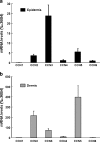
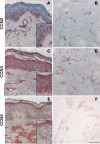
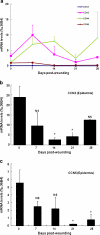
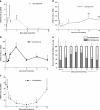
CCN proteins are important modulators of development and function of adult organs. In this study, we examined the localization and expression of the six CCN family members in normal adult human skin and during wound healing in vivo. Transcript and protein expression were studied by laser-capture microdissection-coupled real-time PCR and immunohistochemistry, respectively. Our results demonstrate that CCN1, CCN4, and CCN6 are expressed at relatively low levels in normal human skin. CCN2, CCN3, and CCN5 are the most highly expressed transcripts in the epidermis. CCN3 and CCN5 proteins are prominent in epidermal keratinocytes, whereas CCN2 is primarily expressed in melanocytes. Differential expression within epidermal layers suggests that CCN3 and CCN5 are linked with keratinocyte differentiation. CCN2, CCN3 and CCN5, are the three most highly expressed transcripts in the dermis. Their respective proteins are produced to various extents by dermal fibroblasts, blood vessels, eccrine sweat glands and hair follicles. We find that most CCN family members are temporally and specifically regulated during different phases (inflammation, proliferation, and remodeling) of partial thickness wound repair. By highlighting spatial-temporal regulations of CCN family member expression in relation to cell proliferation and differentiation, our results suggest a diverse range of functions for CCN proteins in both epidermal and dermal cells, and provides a solid reference for interpretation of future studies aimed at understanding the role of CCN proteins in human skin physiology and diseases.
Figures



Similar articles
-
Temperal and spatial expression of CCN1, CCN3, CCN4, CCN5 and CCN6 proteins in the developing postnatal teeth.J Cell Commun Signal. 2023 Jun;17(2):275-285. doi: 10.1007/s12079-023-00758-7. Epub 2023 May 9. J Cell Commun Signal. 2023. PMID: 37160590 Free PMC article.
-
Expression of CCN family of genes in human skin in vivo and alterations by solar-simulated ultraviolet irradiation.J Cell Commun Signal. 2009 Mar;3(1):19-23. doi: 10.1007/s12079-009-0044-8. Epub 2009 Mar 25. J Cell Commun Signal. 2009. PMID: 19319669 Free PMC article.
-
Differential roles of CCN family proteins during osteoblast differentiation: Involvement of Smad and MAPK signaling pathways.Bone. 2011 Nov;49(5):975-89. doi: 10.1016/j.bone.2011.06.033. Epub 2011 Jul 7. Bone. 2011. PMID: 21763478
-
The CCN proteins: important signaling mediators in stem cell differentiation and tumorigenesis.Histol Histopathol. 2010 Jun;25(6):795-806. doi: 10.14670/HH-25.795. Histol Histopathol. 2010. PMID: 20376786 Free PMC article. Review.
-
Targeting CCN Proteins in Rheumatoid Arthritis and Osteoarthritis.Int J Mol Sci. 2021 Apr 21;22(9):4340. doi: 10.3390/ijms22094340. Int J Mol Sci. 2021. PMID: 33919365 Free PMC article. Review.
Cited by
-
CCN2 suppresses catabolic effects of interleukin-1β through α5β1 and αVβ3 integrins in nucleus pulposus cells: implications in intervertebral disc degeneration.J Biol Chem. 2014 Mar 14;289(11):7374-87. doi: 10.1074/jbc.M113.526111. Epub 2014 Jan 24. J Biol Chem. 2014. PMID: 24464580 Free PMC article.
-
CCN proteins: A centralized communication network.J Cell Commun Signal. 2013 Aug;7(3):169-77. doi: 10.1007/s12079-013-0193-7. Epub 2013 Feb 19. J Cell Commun Signal. 2013. PMID: 23420091 Free PMC article.
-
Capturing the finer points of gene expression in psoriasis: beaming in on the CCL19/CCR7 axis.J Invest Dermatol. 2012 Jun;132(6):1535-8. doi: 10.1038/jid.2012.134. J Invest Dermatol. 2012. PMID: 22584500 Free PMC article.
-
Heparin-binding EGF-like growth factor promotes epithelial-mesenchymal transition in human keratinocytes.J Invest Dermatol. 2012 Sep;132(9):2148-57. doi: 10.1038/jid.2012.78. Epub 2012 May 17. J Invest Dermatol. 2012. PMID: 22592159 Free PMC article.
-
A comparison of epithelial-to-mesenchymal transition and re-epithelialization.Semin Cancer Biol. 2012 Oct;22(5-6):471-83. doi: 10.1016/j.semcancer.2012.07.003. Epub 2012 Jul 31. Semin Cancer Biol. 2012. PMID: 22863788 Free PMC article. Review.
References
-
- Falanga V, editor. Cutaneous wound healing. London: Martin Dunitz Ltd; 2001.
LinkOut - more resources
Full Text Sources
Miscellaneous

