Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene
- PMID: 21481789
- PMCID: PMC3077713
- DOI: 10.1016/j.ccr.2011.02.007
Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene
Abstract
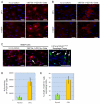
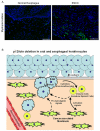
p120-catenin (p120ctn) interacts with E-cadherin, but to our knowledge, no formal proof that p120ctn functions as a bona fide tumor suppressor gene has emerged to date. We report herein that p120ctn loss leads to tumor development in mice. We have generated a conditional knockout model of p120ctn whereby mice develop preneoplastic and neoplastic lesions in the oral cavity, esophagus, and squamous forestomach. Tumor-derived cells secrete granulocyte macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), monocyte chemotactic protein-1 (MCP-1), and tumor necrosis factor-α (TNFα). The tumors contain significant desmoplasia and immune cell infiltration. Immature myeloid cells comprise a significant percentage of the immune cells present and likely participate in fostering a favorable tumor microenvironment, including the activation of fibroblasts.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
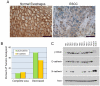
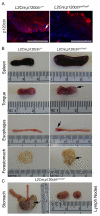

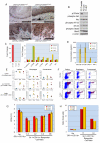
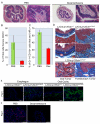
Comment in
-
The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment.Cancer Cell. 2011 Apr 12;19(4):429-31. doi: 10.1016/j.ccr.2011.03.018. Cancer Cell. 2011. PMID: 21481782 Free PMC article.
Similar articles
-
Ocular anterior segment dysgenesis upon ablation of p120 catenin in neural crest cells.Invest Ophthalmol Vis Sci. 2012 Aug 7;53(9):5139-53. doi: 10.1167/iovs.12-9472. Invest Ophthalmol Vis Sci. 2012. PMID: 22736606
-
Altered E-cadherin expression and p120 catenin localization in esophageal squamous cell carcinoma.Ann Surg Oncol. 2007 Nov;14(11):3260-7. doi: 10.1245/s10434-007-9511-8. Epub 2007 Jul 24. Ann Surg Oncol. 2007. PMID: 17647062
-
[Cadherin switching induced by P120-catenin can promote the migration and invasion of oral squamous cell cancer cells].Hua Xi Kou Qiang Yi Xue Za Zhi. 2017 Apr 1;35(2):183-186. doi: 10.7518/hxkq.2017.02.014. Hua Xi Kou Qiang Yi Xue Za Zhi. 2017. PMID: 28682550 Free PMC article. Chinese.
-
Functions of p120ctn in development and disease.Front Biosci (Landmark Ed). 2012 Jan 1;17(2):760-83. doi: 10.2741/3956. Front Biosci (Landmark Ed). 2012. PMID: 22201773 Review.
-
p120-catenin in cancer - mechanisms, models and opportunities for intervention.J Cell Sci. 2013 Aug 15;126(Pt 16):3515-25. doi: 10.1242/jcs.134411. J Cell Sci. 2013. PMID: 23950111 Review.
Cited by
-
The evolution of the cancer niche during multistage carcinogenesis.Nat Rev Cancer. 2013 Jul;13(7):511-8. doi: 10.1038/nrc3536. Epub 2013 Jun 13. Nat Rev Cancer. 2013. PMID: 23760023 Review.
-
Functional analysis of ESRP1/2 gene variants and CTNND1 isoforms in orofacial cleft pathogenesis.bioRxiv [Preprint]. 2024 Jul 2:2024.07.02.601574. doi: 10.1101/2024.07.02.601574. bioRxiv. 2024. Update in: Commun Biol. 2024 Aug 23;7(1):1040. doi: 10.1038/s42003-024-06715-3. PMID: 39005284 Free PMC article. Updated. Preprint.
-
p120 catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression.Prog Mol Biol Transl Sci. 2013;116:409-32. doi: 10.1016/B978-0-12-394311-8.00018-2. Prog Mol Biol Transl Sci. 2013. PMID: 23481205 Free PMC article. Review.
-
A requirement for p120-catenin in the metastasis of invasive ductal breast cancer.J Cell Sci. 2021 Mar 17;134(6):jcs250639. doi: 10.1242/jcs.250639. J Cell Sci. 2021. PMID: 33097605 Free PMC article.
-
Novel epigenetic network biomarkers for early detection of esophageal cancer.Clin Epigenetics. 2022 Feb 14;14(1):23. doi: 10.1186/s13148-022-01243-5. Clin Epigenetics. 2022. PMID: 35164838 Free PMC article.
References
-
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2:637–644. - PubMed
-
- Andl CD, Mizushima T, Nakagawa H, Oyama K, Harada H, Chruma K, Herlyn M, Rustgi AK. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem. 2003;278:1824–1830. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- CA111947/CA/NCI NIH HHS/United States
- P50 CA095103/CA/NCI NIH HHS/United States
- R01-DK069984/DK/NIDDK NIH HHS/United States
- T32-CA115299/CA/NCI NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- P01 CA098101-08/CA/NCI NIH HHS/United States
- R01 CA111947/CA/NCI NIH HHS/United States
- U01 CA143056/CA/NCI NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- R01 CA055724/CA/NCI NIH HHS/United States
- P30-DK050306/DK/NIDDK NIH HHS/United States
- T32 CA115299/CA/NCI NIH HHS/United States
- K99 CA138498/CA/NCI NIH HHS/United States
- F32-CA1308513/CA/NCI NIH HHS/United States
- U01 CA143056-03/CA/NCI NIH HHS/United States
- U01-CA143056/CA/NCI NIH HHS/United States
- 50CA95103/CA/NCI NIH HHS/United States
- P01-CA098101/CA/NCI NIH HHS/United States
- CA055724/CA/NCI NIH HHS/United States
- P01 CA098101/CA/NCI NIH HHS/United States
- P30-CA016520/CA/NCI NIH HHS/United States
- R01 DK069984/DK/NIDDK NIH HHS/United States
- K99/R00-CA138498/CA/NCI NIH HHS/United States
- R00 CA138498/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

