Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial
- PMID: 21481708
- PMCID: PMC3075549
- DOI: 10.1016/S0140-6736(11)60136-0
Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial
Abstract
Background: Cytomegalovirus end-organ disease can be prevented by giving ganciclovir when viraemia is detected in allograft recipients. Values of viral load correlate with development of end-organ disease and are moderated by pre-existing natural immunity. Our aim was to determine whether vaccine-induced immunity could do likewise.
Methods: We undertook a phase-2 randomised placebo controlled trial in adults awaiting kidney or liver transplantation at the Royal Free Hospital, London, UK. Exclusion criteria were pregnancy, receipt of blood products (except albumin) in the previous 3 months, and simultaneous multiorgan transplantation. 70 patients seronegative and 70 seropositive for cytomegalovirus were randomly assigned from a scratch-off randomisation code in a 1:1 ratio to receive either cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant or placebo, each given at baseline, 1 month and 6 months later. If a patient was transplanted, no further vaccinations were given and serial blood samples were tested for cytomegalovirus DNA by real-time quantitative PCR (rtqPCR). Any patient with one blood sample containing more than 3000 cytomegalovirus genomes per mL received ganciclovir until two consecutive undetectable cytomegalovirus DNA measurements. Safety and immunogenicity were coprimary endpoints and were assessed by intention to treat in patients who received at least one dose of vaccine or placebo. This trial is registered with ClinicalTrials.gov, NCT00299260.
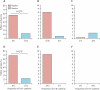
Findings: 67 patients received vaccine and 73 placebo, all of whom were evaluable. Glycoprotein-B antibody titres were significantly increased in both seronegative (geometric mean titre 12,537 (95% CI 6593-23,840) versus 86 (63-118) in recipients of placebo recipients; p<0.0001) and seropositive (118,395; 64,503-217,272) versus 24,682 (17,909-34,017); p<0.0001) recipients of vaccine. In those who developed viraemia after transplantation, glycoprotein-B antibody titres correlated inversely with duration of viraemia (p=0.0022). In the seronegative patients with seropositive donors, the duration of viraemia (p=0.0480) and number of days of ganciclovir treatment (p=0.0287) were reduced in vaccine recipients.
Interpretation: Although cytomegalovirus disease occurs in the context of suppressed cell-mediated immunity post-transplantation, humoral immunity has a role in reduction of cytomegalovirus viraemia. Vaccines containing cytomegalovirus glycoprotein B merit further assessment in transplant recipients.
Funding: National Institute of Allergy and Infectious Diseases, Grant R01AI051355 and Wellcome Trust, Grant 078332.
Sponsor: University College London (UCL).
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
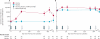

Comment in
-
A cytomegalovirus vaccine tames the troll of transplantation.Lancet. 2011 Apr 9;377(9773):1216-8. doi: 10.1016/S0140-6736(11)60435-2. Lancet. 2011. PMID: 21481691 No abstract available.
Similar articles
-
Seronegative patients vaccinated with cytomegalovirus gB-MF59 vaccine have evidence of neutralising antibody responses against gB early post-transplantation.EBioMedicine. 2019 Dec;50:45-54. doi: 10.1016/j.ebiom.2019.11.005. Epub 2019 Nov 15. EBioMedicine. 2019. PMID: 31735553 Free PMC article.
-
A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial.Lancet Infect Dis. 2012 Apr;12(4):290-9. doi: 10.1016/S1473-3099(11)70344-9. Epub 2012 Jan 10. Lancet Infect Dis. 2012. PMID: 22237175 Clinical Trial.
-
Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: A randomized clinical trial.Vaccine. 2016 Jan 12;34(3):313-9. doi: 10.1016/j.vaccine.2015.11.056. Epub 2015 Dec 2. Vaccine. 2016. PMID: 26657184 Free PMC article. Clinical Trial.
-
Development and evidence for efficacy of CMV glycoprotein B vaccine with MF59 adjuvant.J Clin Virol. 2009 Dec;46 Suppl 4(Suppl 4):S73-6. doi: 10.1016/j.jcv.2009.07.002. Epub 2009 Jul 31. J Clin Virol. 2009. PMID: 19647480 Free PMC article. Review.
-
The next generation recombinant human cytomegalovirus vaccine candidates-beyond gB.Vaccine. 2012 Nov 19;30(49):6980-90. doi: 10.1016/j.vaccine.2012.09.056. Epub 2012 Oct 3. Vaccine. 2012. PMID: 23041121 Review.
Cited by
-
NK Cell Memory to Cytomegalovirus: Implications for Vaccine Development.Vaccines (Basel). 2020 Jul 20;8(3):394. doi: 10.3390/vaccines8030394. Vaccines (Basel). 2020. PMID: 32698362 Free PMC article. Review.
-
Design and analysis of rhesus cytomegalovirus IL-10 mutants as a model for novel vaccines against human cytomegalovirus.PLoS One. 2011;6(11):e28127. doi: 10.1371/journal.pone.0028127. Epub 2011 Nov 21. PLoS One. 2011. PMID: 22132227 Free PMC article.
-
Additive Protection against Congenital Cytomegalovirus Conferred by Combined Glycoprotein B/pp65 Vaccination Using a Lymphocytic Choriomeningitis Virus Vector.Clin Vaccine Immunol. 2017 Jan 5;24(1):e00300-16. doi: 10.1128/CVI.00300-16. Print 2017 Jan. Clin Vaccine Immunol. 2017. PMID: 27795301 Free PMC article.
-
Consensus Definitions of Cytomegalovirus (CMV) Infection and Disease in Transplant Patients Including Resistant and Refractory CMV for Use in Clinical Trials: 2024 Update From the Transplant Associated Virus Infections Forum.Clin Infect Dis. 2024 Sep 26;79(3):787-794. doi: 10.1093/cid/ciae321. Clin Infect Dis. 2024. PMID: 39041385 Free PMC article.
-
Novel trimeric human cytomegalovirus glycoprotein B elicits a high-titer neutralizing antibody response.Vaccine. 2018 Sep 5;36(37):5580-5590. doi: 10.1016/j.vaccine.2018.07.056. Epub 2018 Aug 3. Vaccine. 2018. PMID: 30082162 Free PMC article.
References
-
- Stratton KR, Durch JS, Lawrence RS. Vaccines for the 21st Century (2001) National Academy Press; Washington, DC: 2001. pp. 1–460. - PubMed
-
- Schleiss MR, Heineman TC. Progress toward an elusive goal: current status of cytomegalovirus vaccines. Expert Rev Vaccines. 2005;4:381–406. - PubMed
-
- Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39:233–239. - PubMed
-
- Grundy JE, Super M, Sweny P. Symptomatic cytomegalovirus infection in seropositive kidney recipients: reinfection with donor virus rather than reactivation of recipient virus. Lancet. 1988;332:132–135. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

