Taste receptor signalling - from tongues to lungs
- PMID: 21481196
- PMCID: PMC3704337
- DOI: 10.1111/j.1748-1716.2011.02308.x
Taste receptor signalling - from tongues to lungs
Abstract
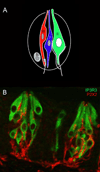
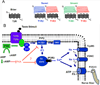
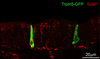
Taste buds are the transducing endorgans of gustation. Each taste bud comprises 50-100 elongated cells, which extend from the basal lamina to the surface of the tongue, where their apical microvilli encounter taste stimuli in the oral cavity. Salts and acids utilize apically located ion channels for transduction, while bitter, sweet and umami (glutamate) stimuli utilize G-protein-coupled receptors (GPCRs) and second-messenger signalling mechanisms. This review will focus on GPCR signalling mechanisms. Two classes of taste GPCRs have been identified, the T1Rs for sweet and umami (glutamate) stimuli and the T2Rs for bitter stimuli. These low affinity GPCRs all couple to the same downstream signalling effectors that include Gβγ activation of phospholipase Cβ2, 1,4,5-inositol trisphosphate mediated release of Ca(2+) from intracellular stores and Ca(2+) -dependent activation of the monovalent selective cation channel, TrpM5. These events lead to membrane depolarization, action potentials and release of ATP as a transmitter to activate gustatory afferents. The Gα subunit, α-gustducin, activates a phosphodiesterase to decrease intracellular cAMP levels, although the precise targets of cAMP have not been identified. With the molecular identification of the taste GPCRs, it has become clear that taste signalling is not limited to taste buds, but occurs in many cell types of the airways. These include solitary chemosensory cells, ciliated epithelial cells and smooth muscle cells. Bitter receptors are most abundantly expressed in the airways, where they respond to irritating chemicals and promote protective airway reflexes, utilizing the same downstream signalling effectors as taste cells.
© 2011 The Author. Acta Physiologica © 2011 Scandinavian Physiological Society.
Conflict of interest statement
Figures
Similar articles
-
Umami taste transduction mechanisms.Am J Clin Nutr. 2009 Sep;90(3):753S-755S. doi: 10.3945/ajcn.2009.27462K. Epub 2009 Jul 1. Am J Clin Nutr. 2009. PMID: 19571214 Free PMC article. Review.
-
The pharmacology and signaling of bitter, sweet, and umami taste sensing.Mol Interv. 2007 Apr;7(2):87-98. doi: 10.1124/mi.7.2.9. Mol Interv. 2007. PMID: 17468389 Review.
-
Expression of taste receptors in solitary chemosensory cells of rodent airways.BMC Pulm Med. 2011 Jan 13;11:3. doi: 10.1186/1471-2466-11-3. BMC Pulm Med. 2011. PMID: 21232137 Free PMC article.
-
Expression of Galpha14 in sweet-transducing taste cells of the posterior tongue.BMC Neurosci. 2008 Nov 13;9:110. doi: 10.1186/1471-2202-9-110. BMC Neurosci. 2008. PMID: 19014514 Free PMC article.
-
Expression of gustducin overlaps with that of type III IP3 receptor in taste buds of the rat soft palate.Chem Senses. 2007 Sep;32(7):689-96. doi: 10.1093/chemse/bjm036. Epub 2007 Jun 12. Chem Senses. 2007. PMID: 17566068
Cited by
-
The aglycone of ginsenoside Rg3 enables glucagon-like peptide-1 secretion in enteroendocrine cells and alleviates hyperglycemia in type 2 diabetic mice.Sci Rep. 2015 Dec 17;5:18325. doi: 10.1038/srep18325. Sci Rep. 2015. PMID: 26675132 Free PMC article.
-
From receptors to the brain: Psychophysical clues to taste physiology.Curr Opin Physiol. 2021 Apr;20:154-158. doi: 10.1016/j.cophys.2020.12.010. Epub 2021 Jan 19. Curr Opin Physiol. 2021. PMID: 33585729 Free PMC article.
-
Chemosensors in the nose: guardians of the airways.Physiology (Bethesda). 2013 Jan;28(1):51-60. doi: 10.1152/physiol.00035.2012. Physiology (Bethesda). 2013. PMID: 23280357 Free PMC article. Review.
-
Chemesthesis and the chemical senses as components of a "chemofensor complex".Chem Senses. 2012 Mar;37(3):201-6. doi: 10.1093/chemse/bjr119. Epub 2011 Dec 30. Chem Senses. 2012. PMID: 22210122 Free PMC article. Review.
-
Taste Receptors Mediate Sinonasal Immunity and Respiratory Disease.Int J Mol Sci. 2017 Feb 17;18(2):437. doi: 10.3390/ijms18020437. Int J Mol Sci. 2017. PMID: 28218655 Free PMC article. Review.
References
-
- Abaffy T, Trubey KR, Chaudhari N. Adenylyl cyclase expression and modulation of cAMP in rat taste cells. Am J Physiol Cell Physiol. 2003;284:C1420–C1428. - PubMed
-
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DC007495/DC/NIDCD NIH HHS/United States
- R01 DC006021/DC/NIDCD NIH HHS/United States
- DC009820/DC/NIDCD NIH HHS/United States
- DC00766/DC/NIDCD NIH HHS/United States
- R01 DC000766-19/DC/NIDCD NIH HHS/United States
- R01 DC007495-05/DC/NIDCD NIH HHS/United States
- R01 DC009820-03/DC/NIDCD NIH HHS/United States
- R01 DC006021-03/DC/NIDCD NIH HHS/United States
- R01 DC000766/DC/NIDCD NIH HHS/United States
- P30 DC004657/DC/NIDCD NIH HHS/United States
- R01 DC007495/DC/NIDCD NIH HHS/United States
- P30DC004657/DC/NIDCD NIH HHS/United States
- DC006021/DC/NIDCD NIH HHS/United States
- R01 DC009820/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

