Extracellular leucine-rich repeats as a platform for receptor/coreceptor complex formation
- PMID: 21464298
- PMCID: PMC3100946
- DOI: 10.1073/pnas.1103556108
Extracellular leucine-rich repeats as a platform for receptor/coreceptor complex formation
Abstract
Receptor kinases with leucine-rich repeat (LRR) extracellular domains form the largest family of receptors in plants. In the few cases for which there is mechanistic information, ligand binding in the extracellular domain often triggers the recruitment of a LRR-coreceptor kinase. The current model proposes that this recruitment is mediated by their respective kinase domains. Here, we show that the extracellular LRR domain of BRI1-ASSOCIATED KINASE1 (BAK1), a coreceptor involved in the disparate processes of cell surface steroid signaling and immunity in plants, is critical for its association with specific ligand-binding LRR-containing receptors. The LRRs of BAK1 thus serve as a platform for the molecular assembly of signal-competent receptors. We propose that this mechanism represents a paradigm for LRR receptor activation in plants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
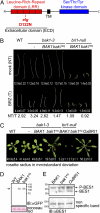
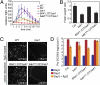

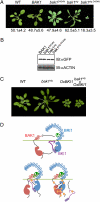
Comment in
-
Direct involvement of leucine-rich repeats in assembling ligand-triggered receptor-coreceptor complexes.Proc Natl Acad Sci U S A. 2011 May 17;108(20):8073-4. doi: 10.1073/pnas.1104057108. Epub 2011 May 5. Proc Natl Acad Sci U S A. 2011. PMID: 21551102 Free PMC article. No abstract available.
Similar articles
-
Assessing the diverse functions of BAK1 and its homologs in arabidopsis, beyond BR signaling and PTI responses.Mol Cells. 2013 Jan;35(1):7-16. doi: 10.1007/s10059-013-2255-3. Epub 2012 Dec 21. Mol Cells. 2013. PMID: 23269431 Free PMC article. Review.
-
Mechanistic basis for the activation of plant membrane receptor kinases by SERK-family coreceptors.Proc Natl Acad Sci U S A. 2018 Mar 27;115(13):3488-3493. doi: 10.1073/pnas.1714972115. Epub 2018 Mar 12. Proc Natl Acad Sci U S A. 2018. PMID: 29531026 Free PMC article.
-
Constitutive Activation of Leucine-Rich Repeat Receptor Kinase Signaling Pathways by BAK1-INTERACTING RECEPTOR-LIKE KINASE3 Chimera.Plant Cell. 2020 Oct;32(10):3311-3323. doi: 10.1105/tpc.20.00138. Epub 2020 Aug 13. Plant Cell. 2020. PMID: 32796127 Free PMC article.
-
Two for all: receptor-associated kinases SOBIR1 and BAK1.Trends Plant Sci. 2014 Feb;19(2):123-32. doi: 10.1016/j.tplants.2013.10.003. Epub 2013 Nov 12. Trends Plant Sci. 2014. PMID: 24238702
-
The growth-defense pivot: crisis management in plants mediated by LRR-RK surface receptors.Trends Biochem Sci. 2014 Oct;39(10):447-56. doi: 10.1016/j.tibs.2014.06.006. Epub 2014 Aug 1. Trends Biochem Sci. 2014. PMID: 25089011 Free PMC article. Review.
Cited by
-
A Mutant Allele Uncouples the Brassinosteroid-Dependent and Independent Functions of BRASSINOSTEROID INSENSITIVE 1.Plant Physiol. 2020 Jan;182(1):669-678. doi: 10.1104/pp.19.00448. Epub 2019 Oct 22. Plant Physiol. 2020. PMID: 31641077 Free PMC article.
-
Direct involvement of leucine-rich repeats in assembling ligand-triggered receptor-coreceptor complexes.Proc Natl Acad Sci U S A. 2011 May 17;108(20):8073-4. doi: 10.1073/pnas.1104057108. Epub 2011 May 5. Proc Natl Acad Sci U S A. 2011. PMID: 21551102 Free PMC article. No abstract available.
-
A sweet story: Bean pod mottle virus transmission dynamics by Mexican bean beetles (Epilachna varivestis).Genome Biol Evol. 2017 Mar;9(3):714-725. doi: 10.1093/gbe/evx033. Epub 2017 Feb 15. Genome Biol Evol. 2017. PMID: 28204501 Free PMC article.
-
Assessing the diverse functions of BAK1 and its homologs in arabidopsis, beyond BR signaling and PTI responses.Mol Cells. 2013 Jan;35(1):7-16. doi: 10.1007/s10059-013-2255-3. Epub 2012 Dec 21. Mol Cells. 2013. PMID: 23269431 Free PMC article. Review.
-
Mechanism of Salt-Induced Self-Compatibility Dissected by Comparative Proteomic Analysis in Brassica napus L.Int J Mol Sci. 2018 Jun 3;19(6):1652. doi: 10.3390/ijms19061652. Int J Mol Sci. 2018. PMID: 29865276 Free PMC article.
References
-
- De Smet I, Voss U, Jürgens G, Beeckman T. Receptor-like kinases shape the plant. Nat Cell Biol. 2009;11:1166–1173. - PubMed
-
- Boller T, Felix G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. - PubMed
-
- Vert G, Nemhauser JL, Geldner N, Hong F, Chory J. Molecular mechanisms of steroid hormone signaling in plants. Annu Rev Cell Dev Biol. 2005;21:177–201. - PubMed
-
- Gómez-Gómez L, Boller T. FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–1011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

