Peroxisome assembly: matrix and membrane protein biogenesis
- PMID: 21464226
- PMCID: PMC3082194
- DOI: 10.1083/jcb.201010022
Peroxisome assembly: matrix and membrane protein biogenesis
Abstract
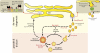
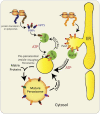
The biogenesis of peroxisomal matrix and membrane proteins is substantially different from the biogenesis of proteins of other subcellular compartments, such as mitochondria and chloroplasts, that are of endosymbiotic origin. Proteins are targeted to the peroxisome matrix through interactions between specific targeting sequences and receptor proteins, followed by protein translocation across the peroxisomal membrane. Recent advances have shed light on the nature of the peroxisomal translocon in matrix protein import and the molecular mechanisms of receptor recycling. Furthermore, the endoplasmic reticulum has been shown to play an important role in peroxisomal membrane protein biogenesis. Defining the molecular events in peroxisome assembly may enhance our understanding of the etiology of human peroxisome biogenesis disorders.
Figures

Similar articles
-
How are peroxisomes formed? The role of the endoplasmic reticulum and peroxins.Trends Plant Sci. 2001 Jun;6(6):256-61. doi: 10.1016/s1360-1385(01)01951-3. Trends Plant Sci. 2001. PMID: 11378467
-
Inactivation of the endoplasmic reticulum protein translocation factor, Sec61p, or its homolog, Ssh1p, does not affect peroxisome biogenesis.Proc Natl Acad Sci U S A. 2001 Oct 9;98(21):12027-31. doi: 10.1073/pnas.221289498. Epub 2001 Oct 2. Proc Natl Acad Sci U S A. 2001. PMID: 11593013 Free PMC article.
-
Import of peroxisomal matrix and membrane proteins.Annu Rev Biochem. 2000;69:399-418. doi: 10.1146/annurev.biochem.69.1.399. Annu Rev Biochem. 2000. PMID: 10966464 Review.
-
Peroxisome biogenesis disorders: molecular basis for impaired peroxisomal membrane assembly: in metabolic functions and biogenesis of peroxisomes in health and disease.Biochim Biophys Acta. 2012 Sep;1822(9):1337-42. doi: 10.1016/j.bbadis.2012.06.004. Epub 2012 Jun 13. Biochim Biophys Acta. 2012. PMID: 22705440 Review.
-
Evaluation of the role of the endoplasmic reticulum-Golgi transit in the biogenesis of peroxisomal membrane proteins in wild type and peroxisome biogenesis mutant CHO cells.Biol Res. 2007;40(2):231-49. doi: 10.4067/s0716-97602007000200014. Epub 2007 Nov 21. Biol Res. 2007. PMID: 18064360
Cited by
-
Dynein light chain interaction with the peroxisomal import docking complex modulates peroxisome biogenesis in yeast.J Cell Sci. 2013 Oct 15;126(Pt 20):4698-706. doi: 10.1242/jcs.129056. Epub 2013 Aug 13. J Cell Sci. 2013. PMID: 23943868 Free PMC article.
-
Conserved targeting information in mammalian and fungal peroxisomal tail-anchored proteins.Sci Rep. 2015 Dec 2;5:17420. doi: 10.1038/srep17420. Sci Rep. 2015. PMID: 26627908 Free PMC article.
-
A critical appraisal of quantitative studies of protein degradation in the framework of cellular proteostasis.Biochem Res Int. 2012;2012:823597. doi: 10.1155/2012/823597. Epub 2012 Oct 21. Biochem Res Int. 2012. PMID: 23119163 Free PMC article.
-
Pex3-anchored Atg36 tags peroxisomes for degradation in Saccharomyces cerevisiae.EMBO J. 2012 Jun 29;31(13):2852-68. doi: 10.1038/emboj.2012.151. Epub 2012 May 29. EMBO J. 2012. PMID: 22643220 Free PMC article.
-
Molecular insights into intracellular RNA localization.Int Rev Cell Mol Biol. 2013;302:1-39. doi: 10.1016/B978-0-12-407699-0.00001-7. Int Rev Cell Mol Biol. 2013. PMID: 23351709 Free PMC article. Review.
References
-
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. 2002. Molecular Biology of the Cell. Garland Science, New York: 1616 pp
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

