Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells
- PMID: 21454756
- PMCID: PMC3322670
- DOI: 10.1161/CIRCRESAHA.110.239046
Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells
Abstract
Rationale: The ability of the adult heart to generate new myocytes after injury is not established.
Objective: Our purpose was to determine whether the adult heart has the capacity to generate new myocytes after injury, and to gain insight into their source.
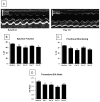
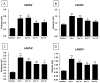
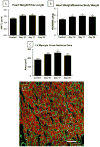
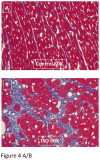
Methods and results: Cardiac injury was induced in the adult feline heart by infusing isoproterenol (ISO) for 10 days via minipumps, and then animals were allowed to recover for 7 or 28 days. Cardiac function was measured with echocardiography, and proliferative cells were identified by nuclear incorporation of 5-bromodeoxyuridine (BrdU; 7-day minipump infusion). BrdU was infused for 7 days before euthanasia at days 10, 17, and 38 or during injury and animals euthanized at day 38. ISO caused reduction in cardiac function with evidence of myocyte loss from necrosis. During this injury phase there was a significant increase in the number of proliferative cells in the atria and ventricle, but there was no increase in BrdU+ myocytes. cKit+ cardiac progenitor cells were BrdU labeled during injury. During the first 7 days of recovery there was a significant reduction in cellular proliferation (BrdU incorporation) but a significant increase in BrdU+ myocytes. There was modest improvement in cardiac structure and function during recovery. At day 38, overall cell proliferation was not different than control, but increased numbers of BrdU+ myocytes were found when BrdU was infused during injury.
Conclusions: These studies suggest that ISO injury activates cardiac progenitor cells that can differentiate into new myocytes during cardiac repair.
Figures

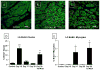
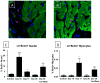
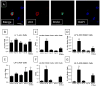

Comment in
-
Curiosity killed the cat and found new myocytes.Circ Res. 2011 May 13;108(10):1158-9. doi: 10.1161/CIRCRESAHA.111.245571. Circ Res. 2011. PMID: 21566219 No abstract available.
Similar articles
-
Autologous c-Kit+ Mesenchymal Stem Cell Injections Provide Superior Therapeutic Benefit as Compared to c-Kit+ Cardiac-Derived Stem Cells in a Feline Model of Isoproterenol-Induced Cardiomyopathy.Clin Transl Sci. 2015 Oct;8(5):425-31. doi: 10.1111/cts.12251. Epub 2015 Feb 11. Clin Transl Sci. 2015. PMID: 25684108 Free PMC article.
-
Acute Catecholamine Exposure Causes Reversible Myocyte Injury Without Cardiac Regeneration.Circ Res. 2016 Sep 16;119(7):865-79. doi: 10.1161/CIRCRESAHA.116.308687. Epub 2016 Jul 26. Circ Res. 2016. PMID: 27461939 Free PMC article.
-
DNA resynthesis and binucleated metamorphosis in cardiac muscle cells after isoproterenol-induced injury: bromodeoxyuridine immunohistochemistry.Am J Cardiovasc Pathol. 1995;5(1):49-54. Am J Cardiovasc Pathol. 1995. PMID: 8838156
-
Hope for a broken heart?Cell. 2003 Sep 19;114(6):658-9. doi: 10.1016/s0092-8674(03)00718-9. Cell. 2003. PMID: 14505565 Review.
-
"AKT"ing lessons for stem cells: regulation of cardiac myocyte and progenitor cell proliferation.Trends Cardiovasc Med. 2007 Oct;17(7):235-40. doi: 10.1016/j.tcm.2007.08.003. Trends Cardiovasc Med. 2007. PMID: 17936205 Free PMC article. Review.
Cited by
-
A strong regenerative ability of cardiac stem cells derived from neonatal hearts.Circulation. 2012 Sep 11;126(11 Suppl 1):S46-53. doi: 10.1161/CIRCULATIONAHA.111.084699. Circulation. 2012. PMID: 22965993 Free PMC article.
-
Advances in stem cell therapy for cardiovascular disease (Review).Int J Mol Med. 2016 Jul;38(1):23-9. doi: 10.3892/ijmm.2016.2607. Epub 2016 May 25. Int J Mol Med. 2016. PMID: 27220939 Free PMC article. Review.
-
Pathologic Stimulus Determines Lineage Commitment of Cardiac C-kit+ Cells.Circulation. 2017 Dec 12;136(24):2359-2372. doi: 10.1161/CIRCULATIONAHA.117.030137. Epub 2017 Oct 11. Circulation. 2017. PMID: 29021323 Free PMC article.
-
TNF receptor signaling inhibits cardiomyogenic differentiation of cardiac stem cells and promotes a neuroadrenergic-like fate.Am J Physiol Heart Circ Physiol. 2016 Nov 1;311(5):H1189-H1201. doi: 10.1152/ajpheart.00904.2015. Epub 2016 Sep 2. Am J Physiol Heart Circ Physiol. 2016. PMID: 27591224 Free PMC article.
-
Left atrial appendages from adult hearts contain a reservoir of diverse cardiac progenitor cells.PLoS One. 2013;8(3):e59228. doi: 10.1371/journal.pone.0059228. Epub 2013 Mar 12. PLoS One. 2013. PMID: 23555001 Free PMC article.
References
-
- Zak R. Development and proliferative capacity of cardiac muscle cells. Circ Res. 1974;35(suppl II):17–26. - PubMed
-
- Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res. 1998;83:15–26. - PubMed
-
- Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–14. - PubMed
-
- Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, D’Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, Del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL088243/HL/NHLBI NIH HHS/United States
- HL033921/HL/NHLBI NIH HHS/United States
- HL095322/HL/NHLBI NIH HHS/United States
- HL089312/HL/NHLBI NIH HHS/United States
- F30 HL095322/HL/NHLBI NIH HHS/United States
- R01 HL090979/HL/NHLBI NIH HHS/United States
- R01 HL090979-01/HL/NHLBI NIH HHS/United States
- R01 HL089312-05/HL/NHLBI NIH HHS/United States
- R37 HL033921/HL/NHLBI NIH HHS/United States
- R01 HL033921/HL/NHLBI NIH HHS/United States
- HL091799/HL/NHLBI NIH HHS/United States
- R01 HL089312/HL/NHLBI NIH HHS/United States
- P01 HL091799/HL/NHLBI NIH HHS/United States
- R01 HL033921-24/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

