MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo
- PMID: 21444814
- PMCID: PMC3076836
- DOI: 10.1073/pnas.1016758108
MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo
Abstract
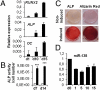
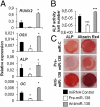
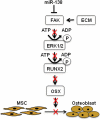
Elucidating the molecular mechanisms that regulate human stromal (mesenchymal) stem cell (hMSC) differentiation into osteogenic lineage is important for the development of anabolic therapies for treatment of osteoporosis. MicroRNAs (miRNAs) are short, noncoding RNAs that act as key regulators of diverse biological processes by mediating translational repression or mRNA degradation of their target genes. Here, we show that miRNA-138 (miR-138) modulates osteogenic differentiation of hMSCs. miRNA array profiling and further validation by quantitative RT-PCR (qRT-PCR) revealed that miR-138 was down-regulated during osteoblast differentiation of hMSCs. Overexpression of miR-138 inhibited osteoblast differentiation of hMSCs in vitro, whereas inhibition of miR-138 function by antimiR-138 promoted expression of osteoblast-specific genes, alkaline phosphatase (ALP) activity, and matrix mineralization. Furthermore, overexpression of miR-138 reduced ectopic bone formation in vivo by 85%, and conversely, in vivo bone formation was enhanced by 60% when miR-138 was antagonized. Target prediction analysis and experimental validation by luciferase 3' UTR reporter assay confirmed focal adhesion kinase, a kinase playing a central role in promoting osteoblast differentiation, as a bona fide target of miR-138. We show that miR-138 attenuates bone formation in vivo, at least in part by inhibiting the focal adhesion kinase signaling pathway. Our findings suggest that pharmacological inhibition of miR-138 by antimiR-138 could represent a therapeutic strategy for enhancing bone formation in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
MicroRNA-34a inhibits osteoblast differentiation and in vivo bone formation of human stromal stem cells.Stem Cells. 2014 Apr;32(4):902-12. doi: 10.1002/stem.1615. Stem Cells. 2014. PMID: 24307639
-
miR-542-3p suppresses osteoblast cell proliferation and differentiation, targets BMP-7 signaling and inhibits bone formation.Cell Death Dis. 2014 Feb 6;5(2):e1050. doi: 10.1038/cddis.2014.4. Cell Death Dis. 2014. PMID: 24503542 Free PMC article.
-
MicroRNA-579-3p promotes the progression of osteoporosis by inhibiting osteogenic differentiation of mesenchymal stem cells through regulating Sirt1.Eur Rev Med Pharmacol Sci. 2019 Aug;23(16):6791-6799. doi: 10.26355/eurrev_201908_18717. Eur Rev Med Pharmacol Sci. 2019. PMID: 31486477
-
MicroRNA functions in osteogenesis and dysfunctions in osteoporosis.Curr Osteoporos Rep. 2013 Jun;11(2):72-82. doi: 10.1007/s11914-013-0143-6. Curr Osteoporos Rep. 2013. PMID: 23605904 Free PMC article. Review.
-
Regulation of Wnt signaling by non-coding RNAs during osteoblast differentiation.Differentiation. 2022 Nov-Dec;128:57-66. doi: 10.1016/j.diff.2022.10.003. Epub 2022 Oct 15. Differentiation. 2022. PMID: 36370525 Review.
Cited by
-
MiR-539-3p impairs osteogenesis by suppressing Wnt interaction with LRP-6 co-receptor and subsequent inhibition of Akap-3 signaling pathway.Front Endocrinol (Lausanne). 2022 Sep 29;13:977347. doi: 10.3389/fendo.2022.977347. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36267566 Free PMC article.
-
Vitamin D and microRNAs in bone.Crit Rev Eukaryot Gene Expr. 2013;23(3):195-214. doi: 10.1615/critreveukaryotgeneexpr.2013007147. Crit Rev Eukaryot Gene Expr. 2013. PMID: 23879537 Free PMC article. Review.
-
Human bone marrow stromal cell confluence: effects on cell characteristics and methods of assessment.Cytotherapy. 2015 Jul;17(7):897-911. doi: 10.1016/j.jcyt.2015.03.607. Epub 2015 Apr 14. Cytotherapy. 2015. PMID: 25882666 Free PMC article.
-
Mechanobiological Feedback in Pulmonary Vascular Disease.Front Physiol. 2018 Jul 25;9:951. doi: 10.3389/fphys.2018.00951. eCollection 2018. Front Physiol. 2018. PMID: 30090065 Free PMC article. Review.
-
Use of adult mesenchymal stromal cells in tissue repair: impact of physical exercise.Am J Physiol Cell Physiol. 2019 Oct 1;317(4):C642-C654. doi: 10.1152/ajpcell.00530.2018. Epub 2019 Jun 26. Am J Physiol Cell Physiol. 2019. PMID: 31241985 Free PMC article. Review.
References
-
- Jaiswal RK, et al. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. - PubMed
-
- Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
-
- Abdallah BM, et al. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochem Biophys Res Commun. 2005;326:527–538. - PubMed
-
- Abdallah BM, Kassem M. Human mesenchymal stem cells: From basic biology to clinical applications. Gene Ther. 2008;15:109–116. - PubMed
-
- Xiao G, et al. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275:4453–4459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

