Human epidermal growth factor receptor (EGFR) aligned on the plasma membrane adopts key features of Drosophila EGFR asymmetry
- PMID: 21444717
- PMCID: PMC3133235
- DOI: 10.1128/MCB.01431-10
Human epidermal growth factor receptor (EGFR) aligned on the plasma membrane adopts key features of Drosophila EGFR asymmetry
Abstract
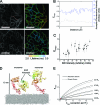
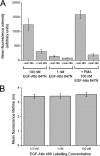
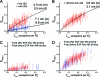
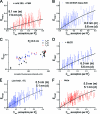
The ability of epidermal growth factor receptor (EGFR) to control cell fate is defined by its affinity for ligand. Current models suggest that ligand-binding heterogeneity arises from negative cooperativity in signaling receptor dimers, for which the asymmetry of the extracellular region of the Drosophila EGFR has recently provided a structural basis. However, no asymmetry is apparent in the isolated extracellular region of the human EGFR. Human EGFR also differs from the Drosophila EGFR in that negative cooperativity is found only in full-length receptors in cells. To gain structural insights into the human EGFR in situ, we developed an approach based on quantitative Förster resonance energy transfer (FRET) imaging, combined with Monte Carlo and molecular dynamics simulations, to probe receptor conformation in epithelial cells. We experimentally demonstrate a high-affinity ligand-binding human EGFR conformation consistent with the extracellular region aligned flat on the plasma membrane. We explored the relevance of this conformation to ligand-binding heterogeneity and found that the asymmetry of this structure shares key features with that of the Drosophila EGFR, suggesting that the structural basis for negative cooperativity is conserved from invertebrates to humans but that in human EGFR the extracellular region asymmetry requires interactions with the plasma membrane.
Figures

Similar articles
-
Human epidermal growth factor receptor (HER1) aligned on the plasma membrane adopts key features of Drosophila EGFR asymmetry.Biochem Soc Trans. 2012 Feb;40(1):184-8. doi: 10.1042/BST20110692. Biochem Soc Trans. 2012. PMID: 22260687
-
A single ligand is sufficient to activate EGFR dimers.Proc Natl Acad Sci U S A. 2012 Jul 3;109(27):10861-6. doi: 10.1073/pnas.1201114109. Epub 2012 Jun 14. Proc Natl Acad Sci U S A. 2012. PMID: 22699492 Free PMC article.
-
Structural basis for negative cooperativity in growth factor binding to an EGF receptor.Cell. 2010 Aug 20;142(4):568-79. doi: 10.1016/j.cell.2010.07.015. Cell. 2010. PMID: 20723758 Free PMC article.
-
A tale of the epidermal growth factor receptor: The quest for structural resolution on cells.Methods. 2016 Feb 15;95:86-93. doi: 10.1016/j.ymeth.2015.10.009. Epub 2015 Oct 17. Methods. 2016. PMID: 26484734 Review.
-
Structure-function relationships and supramolecular organization of the EGFR (epidermal growth factor receptor) on the cell surface.Biochem Soc Trans. 2014 Feb;42(1):114-9. doi: 10.1042/BST20130236. Biochem Soc Trans. 2014. PMID: 24450637 Review.
Cited by
-
Compounds identified by virtual docking to a tetrameric EGFR extracellular domain can modulate Grb2 internalization.BMC Cancer. 2015 May 28;15:436. doi: 10.1186/s12885-015-1415-6. BMC Cancer. 2015. PMID: 26016476 Free PMC article.
-
EGFR oligomerization organizes kinase-active dimers into competent signalling platforms.Nat Commun. 2016 Oct 31;7:13307. doi: 10.1038/ncomms13307. Nat Commun. 2016. PMID: 27796308 Free PMC article.
-
Drug-resistant EGFR mutations promote lung cancer by stabilizing interfaces in ligand-free kinase-active EGFR oligomers.Nat Commun. 2024 Mar 19;15(1):2130. doi: 10.1038/s41467-024-46284-x. Nat Commun. 2024. PMID: 38503739 Free PMC article.
-
Ligand binding and dynamics of the monomeric epidermal growth factor receptor ectodomain.Proteins. 2013 Nov;81(11):1931-43. doi: 10.1002/prot.24339. Epub 2013 Aug 19. Proteins. 2013. PMID: 23760854 Free PMC article.
-
A structural perspective on the regulation of the epidermal growth factor receptor.Annu Rev Biochem. 2015;84:739-64. doi: 10.1146/annurev-biochem-060614-034402. Epub 2015 Jan 26. Annu Rev Biochem. 2015. PMID: 25621509 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
