Improved insulin sensitivity by GLUT12 overexpression in mice
- PMID: 21441439
- PMCID: PMC3292321
- DOI: 10.2337/db11-0033
Improved insulin sensitivity by GLUT12 overexpression in mice
Abstract
Objective: Evidence suggests that insulin-sensitive glucose transporters (GLUTs) other than GLUT4 may exist. To investigate whether GLUT12 may represent another insulin-sensitive GLUT, transgenic (TG) mice that overexpress GLUT12 were characterized.
Research design and methods: TG mice that overexpressed GLUT12 under a β-actin promoter were generated. Glucose metabolism in TG and wild-type control mice was compared using glucose and insulin tolerance tests and hyperinsulinemic-euglycemic clamps. In addition, basal and insulin-stimulated glucose clearance rates into insulin-sensitive peripheral tissues were measured using [(3)H]-2-deoxy-D-glucose.
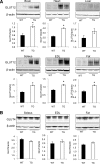
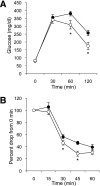
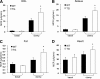
Results: GLUT12 was overexpressed by 40-75% in TG compared with wild-type mice in insulin-sensitive tissues with no change in GLUT4 content. Body weight and fasting blood glucose did not differ between wild-type and TG mice; however, insulin concentrations were reduced in TG mice. Enhanced oral glucose tolerance was noted in TG mice by a reduced blood glucose excursion compared with wild-type mice (P < 0.05). Enhanced insulin sensitivity was noted by a greater decrease in blood glucose in TG mice during insulin tolerance testing. Hyperinsulinemic-euglycemic clamps confirmed enhanced insulin sensitivity in GLUT12-overexpressing mice (P < 0.01). Tissues of TG mice exhibited normal basal glucose clearance rates; however, under insulin-stimulated conditions, glucose clearance was significantly increased (P < 0.01) in tissues of TG mice.
Conclusions: Increased expression of GLUT12 results in improved whole-body insulin sensitivity mediated by an increased glucose clearance rate in insulin-responsive tissues under insulin-stimulated, but not basal, conditions. These findings provide evidence that GLUT12 represents a novel, second insulin-sensitive GLUT.
Figures
Similar articles
-
GLUT12 functions as a basal and insulin-independent glucose transporter in the heart.Biochim Biophys Acta. 2013 Jan;1832(1):121-7. doi: 10.1016/j.bbadis.2012.09.013. Epub 2012 Oct 4. Biochim Biophys Acta. 2013. PMID: 23041416
-
Chronic heart failure selectively induces regional heterogeneity of insulin-responsive glucose transporters.Am J Physiol Regul Integr Comp Physiol. 2011 Nov;301(5):R1300-6. doi: 10.1152/ajpregu.00822.2010. Epub 2011 Aug 17. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21849635 Free PMC article.
-
Insulin-stimulated translocation of glucose transporter (GLUT) 12 parallels that of GLUT4 in normal muscle.J Clin Endocrinol Metab. 2009 Sep;94(9):3535-42. doi: 10.1210/jc.2009-0162. Epub 2009 Jun 23. J Clin Endocrinol Metab. 2009. PMID: 19549745 Free PMC article.
-
The glucose transporter GLUT12, a new actor in obesity and cancer.J Physiol Biochem. 2024 May 10. doi: 10.1007/s13105-024-01028-9. Online ahead of print. J Physiol Biochem. 2024. PMID: 38727993 Review.
-
The facilitative glucose transporter GLUT12: what do we know and what would we like to know?J Physiol Biochem. 2013 Jun;69(2):325-33. doi: 10.1007/s13105-012-0213-8. Epub 2012 Oct 3. J Physiol Biochem. 2013. PMID: 23385668 Review. No abstract available.
Cited by
-
Antimetabolic Effects of Polyphenols in Breast Cancer Cells: Focus on Glucose Uptake and Metabolism.Front Nutr. 2018 Apr 16;5:25. doi: 10.3389/fnut.2018.00025. eCollection 2018. Front Nutr. 2018. PMID: 29713632 Free PMC article. Review.
-
Phylogenesis and Biological Characterization of a New Glucose Transporter in the Chicken (Gallus gallus), GLUT12.PLoS One. 2015 Oct 2;10(10):e0139517. doi: 10.1371/journal.pone.0139517. eCollection 2015. PLoS One. 2015. PMID: 26431526 Free PMC article.
-
SLC2A12 of SLC2 Gene Family in Bird Provides Functional Compensation for the Loss of SLC2A4 Gene in Other Vertebrates.Mol Biol Evol. 2021 Apr 13;38(4):1276-1291. doi: 10.1093/molbev/msaa286. Mol Biol Evol. 2021. PMID: 33316072 Free PMC article.
-
Expression of conventional and novel glucose transporters, GLUT1, -9, -10, and -12, in vascular smooth muscle cells.Am J Physiol Cell Physiol. 2013 Mar;304(6):C574-89. doi: 10.1152/ajpcell.00275.2012. Epub 2013 Jan 9. Am J Physiol Cell Physiol. 2013. PMID: 23302780 Free PMC article.
-
Modeling the effect of cigarette smoke on hexose utilization in spermatocytes.Reprod Sci. 2015 Jan;22(1):94-101. doi: 10.1177/1933719114533727. Epub 2014 May 6. Reprod Sci. 2015. PMID: 24803506 Free PMC article.
References
-
- Katz EB, Stenbit AE, Hatton K, DePinho R, Charron MJ. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature 1995;377:151–155 - PubMed
-
- Rogers S, Macheda ML, Docherty SE, et al. Identification of a novel glucose transporter-like protein-GLUT-12. Am J Physiol Endocrinol Metab 2002;282:E733–E738 - PubMed
-
- Rogers S, Chandler JD, Clarke AL, Petrou S, Best JD. Glucose transporter GLUT12-functional characterization in Xenopus laevis oocytes. Biochem Biophys Res Commun 2003;308:422–426 - PubMed
-
- Verhey KJ, Birnbaum MJ. A Leu-Leu sequence is essential for COOH-terminal targeting signal of GLUT4 glucose transporter in fibroblasts. J Biol Chem 1994;269:2353–2356 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

