Development of an attenuated interleukin-2 fusion protein that can be activated by tumour-expressed proteases
- PMID: 21426339
- PMCID: PMC3088983
- DOI: 10.1111/j.1365-2567.2011.03428.x
Development of an attenuated interleukin-2 fusion protein that can be activated by tumour-expressed proteases
Abstract
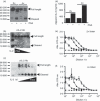
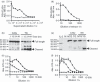
The ability to alter the cytokine microenvironment has the potential to shape immune responses in many physiological settings, including the immunotherapy of tumours. We set out to develop a general approach in which cytokines could be functionally attenuated until activated. We report the development and initial characterization of fusion proteins in which human or mouse interleukin-2 (IL-2), a potent growth factor for immune cells, is joined to a specific IL-2 inhibitory binding component separated by a protease site. The rationale is that upon cleavage by a protease the cytokine is free to dissociate from the inhibitory component and becomes biologically more available. We describe the successful development of two attenuation strategies using specific binding: the first uses the mouse IL-2 receptor alpha chain as the inhibitory binding component whereas the second employs a human antibody fragment (scFv) reactive with human IL-2. We demonstrated that the fusion proteins containing a prostate-specific antigen or a matrix metalloproteinase (MMP) protease cleavage site are markedly attenuated in the intact fusion protein but had enhanced bioactivity of IL-2 in vitro when cleaved. Further, we showed that a fusion protein composed of the IL-2/IL-2 receptor alpha chain with an MMP cleavage site reduced tumour growth in vivo in a peritoneal mouse tumour model. This general strategy should be applicable to other proteases and immune modulators allowing site-specific activation of immunomodulators while reducing unwanted side-effects.
© 2011 The Authors. Immunology © 2011 Blackwell Publishing Ltd.
Figures



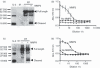
Similar articles
-
Development of an Interleukin-12 Fusion Protein That Is Activated by Cleavage with Matrix Metalloproteinase 9.J Interferon Cytokine Res. 2019 Apr;39(4):233-245. doi: 10.1089/jir.2018.0129. Epub 2019 Mar 8. J Interferon Cytokine Res. 2019. PMID: 30848689 Free PMC article.
-
ALKS 4230: a novel engineered IL-2 fusion protein with an improved cellular selectivity profile for cancer immunotherapy.J Immunother Cancer. 2020 Apr;8(1):e000673. doi: 10.1136/jitc-2020-000673. J Immunother Cancer. 2020. PMID: 32317293 Free PMC article.
-
An antibody fusion protein for cancer immunotherapy mimicking IL-15 trans-presentation at the tumor site.Mol Cancer Ther. 2012 Jun;11(6):1279-88. doi: 10.1158/1535-7163.MCT-12-0019. Epub 2012 Apr 6. Mol Cancer Ther. 2012. PMID: 22491823
-
Increasing the biological activity of IL-2 and IL-15 through complexing with anti-IL-2 mAbs and IL-15Rα-Fc chimera.Immunol Lett. 2014 May-Jun;159(1-2):1-10. doi: 10.1016/j.imlet.2014.01.017. Epub 2014 Feb 7. Immunol Lett. 2014. PMID: 24512738 Review.
-
Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target?Cancer Sci. 2019 Jul;110(7):2080-2089. doi: 10.1111/cas.14069. Epub 2019 Jun 18. Cancer Sci. 2019. PMID: 31102428 Free PMC article. Review.
Cited by
-
Next-generation cytokines for cancer immunotherapy.Antib Ther. 2021 Jun 25;4(2):123-133. doi: 10.1093/abt/tbab014. eCollection 2021 Apr. Antib Ther. 2021. PMID: 34263141 Free PMC article. Review.
-
Tumor-conditional IL-15 pro-cytokine reactivates anti-tumor immunity with limited toxicity.Cell Res. 2021 Nov;31(11):1190-1198. doi: 10.1038/s41422-021-00543-4. Epub 2021 Aug 10. Cell Res. 2021. PMID: 34376814 Free PMC article.
-
Modifying the cancer-immune set point using vaccinia virus expressing re-designed interleukin-2.Nat Commun. 2018 Nov 8;9(1):4682. doi: 10.1038/s41467-018-06954-z. Nat Commun. 2018. PMID: 30410056 Free PMC article.
-
Development of an Interleukin-12 Fusion Protein That Is Activated by Cleavage with Matrix Metalloproteinase 9.J Interferon Cytokine Res. 2019 Apr;39(4):233-245. doi: 10.1089/jir.2018.0129. Epub 2019 Mar 8. J Interferon Cytokine Res. 2019. PMID: 30848689 Free PMC article.
-
Development of oncolytic virotherapy: from genetic modification to combination therapy.Front Med. 2020 Apr;14(2):160-184. doi: 10.1007/s11684-020-0750-4. Epub 2020 Mar 7. Front Med. 2020. PMID: 32146606 Free PMC article. Review.
References
-
- Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–15. - PubMed
-
- Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. - PubMed
-
- Parmiani G, De Filippo A, Novellino L, Castelli C. Unique human tumor antigens: immunobiology and use in clinical trials. J Immunol. 2007;178:1975–9. - PubMed
-
- Slingluff CL, Jr, Chianese-Bullock KA, Bullock TN, et al. Immunity to melanoma antigens: from self-tolerance to immunotherapy. Adv Immunol. 2006;90:243–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

