Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders
- PMID: 21423522
- PMCID: PMC3059681
- DOI: 10.3389/fnsyn.2010.00136
Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders
Abstract
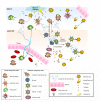
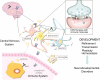
Although the brain has classically been considered "immune-privileged", current research suggests an extensive communication between the immune and nervous systems in both health and disease. Recent studies demonstrate that immune molecules are present at the right place and time to modulate the development and function of the healthy and diseased central nervous system (CNS). Indeed, immune molecules play integral roles in the CNS throughout neural development, including affecting neurogenesis, neuronal migration, axon guidance, synapse formation, activity-dependent refinement of circuits, and synaptic plasticity. Moreover, the roles of individual immune molecules in the nervous system may change over development. This review focuses on the effects of immune molecules on neuronal connections in the mammalian central nervous system - specifically the roles for MHCI and its receptors, complement, and cytokines on the function, refinement, and plasticity of geniculate, cortical and hippocampal synapses, and their relationship to neurodevelopmental disorders. These functions for immune molecules during neural development suggest that they could also mediate pathological responses to chronic elevations of cytokines in neurodevelopmental disorders, including autism spectrum disorders (ASD) and schizophrenia.
Keywords: autism; complement; cytokine; major histocompatibility complex; plasticity; refinement; schizophrenia; synapse.
Figures

Similar articles
-
Major histocompatibility complex I in brain development and schizophrenia.Biol Psychiatry. 2014 Feb 15;75(4):262-8. doi: 10.1016/j.biopsych.2013.10.003. Epub 2013 Oct 10. Biol Psychiatry. 2014. PMID: 24199663 Free PMC article. Review.
-
SynCAMs extend their functions beyond the synapse.Eur J Neurosci. 2014 Jun;39(11):1752-60. doi: 10.1111/ejn.12544. Epub 2014 Mar 15. Eur J Neurosci. 2014. PMID: 24628990 Review.
-
Complement System in Brain Architecture and Neurodevelopmental Disorders.Front Neurosci. 2020 Feb 5;14:23. doi: 10.3389/fnins.2020.00023. eCollection 2020. Front Neurosci. 2020. PMID: 32116493 Free PMC article. Review.
-
MHC class I molecules are present both pre- and postsynaptically in the visual cortex during postnatal development and in adulthood.Proc Natl Acad Sci U S A. 2010 Sep 28;107(39):16999-7004. doi: 10.1073/pnas.1006087107. Epub 2010 Sep 13. Proc Natl Acad Sci U S A. 2010. PMID: 20837535 Free PMC article.
-
Major histocompatibility complex class I proteins in brain development and plasticity.Trends Neurosci. 2012 Nov;35(11):660-70. doi: 10.1016/j.tins.2012.08.001. Epub 2012 Aug 30. Trends Neurosci. 2012. PMID: 22939644 Free PMC article. Review.
Cited by
-
CNF1 improves astrocytic ability to support neuronal growth and differentiation in vitro.PLoS One. 2012;7(4):e34115. doi: 10.1371/journal.pone.0034115. Epub 2012 Apr 16. PLoS One. 2012. PMID: 22523545 Free PMC article.
-
The Effect of TNF-alpha rs1800629 Polymorphism on White Matter Structures and Memory Function in Patients With Schizophrenia: A Pilot Study.Psychiatry Investig. 2022 Dec;19(12):1027-1036. doi: 10.30773/pi.2021.0326. Epub 2022 Dec 22. Psychiatry Investig. 2022. PMID: 36588437 Free PMC article.
-
Maternal Mid-Gestation Cytokine Dysregulation in Mothers of Children with Autism Spectrum Disorder.J Autism Dev Disord. 2022 Sep;52(9):3919-3932. doi: 10.1007/s10803-021-05271-7. Epub 2021 Sep 9. J Autism Dev Disord. 2022. PMID: 34505185 Free PMC article.
-
Transcutaneous Vagus Nerve Stimulation: A Promising Method for Treatment of Autism Spectrum Disorders.Front Neurosci. 2017 Jan 20;10:609. doi: 10.3389/fnins.2016.00609. eCollection 2016. Front Neurosci. 2017. PMID: 28163670 Free PMC article.
-
Tumor necrosis factor-α and -β genetic polymorphisms as a risk factor in Saudi patients with schizophrenia.Neuropsychiatr Dis Treat. 2017 Apr 12;13:1081-1088. doi: 10.2147/NDT.S131144. eCollection 2017. Neuropsychiatr Dis Treat. 2017. PMID: 28442912 Free PMC article.
References
-
- Abbas A, Lichtman A. H., Pober J. (2000). Cellular and Molecular Immunology. Philadelphia, PA: W.E. Saunders Company
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

