Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors
- PMID: 21406401
- PMCID: PMC3077882
- DOI: 10.1158/0008-5472.CAN-10-2277
Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors
Abstract
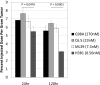
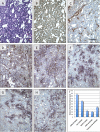
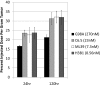
Antibody drugs are widely used in cancer therapy, but conditions to maximize tumor penetration and efficacy have yet to be fully elucidated. In this study, we investigated the impact of antibody binding affinity on tumor targeting and penetration with affinity variants that recognize the same epitope. Specifically, we compared four derivatives of the C6.5 monoclonal antibody (mAb), which recognizes the same HER2 epitope (monovalent K(D) values ranging from 270 to 0.56 nmol/L). Moderate affinity was associated with the highest tumor accumulation at 24 and 120 hours after intravenous injection, whereas high affinity was found to produce the lowest tumor accumulation. Highest affinity mAbs were confined to the perivascular space of tumors with an average penetration of 20.4 ± 7.5 μm from tumor blood vessels. Conversely, lowest affinity mAbs exhibited a broader distribution pattern with an average penetration of 84.8 ± 12.8 μm. In vitro internalization assays revealed that antibody internalization and catabolism generally increased with affinity, plateauing once the rate of HER2 internalization exceeded the rate of antibody dissociation. Effects of internalization and catabolism on tumor targeting were further examined using antibodies of moderate (C6.5) or high-affinity (trastuzumab), labeled with residualizing ((111)In-labeled) or nonresidualizing ((125)I-labeled) radioisotopes. Significant amounts of antibody of both affinities were degraded by tumors in vivo. Furthermore, moderate- to high-affinity mAbs targeting the same HER2 epitope with monovalent affinity above 23 nmol/L had equal tumor accumulation of residualizing radiolabel over 120 hours. Results indicated equal tumor exposure, suggesting that mAb penetration and retention in tumors reflected affinity-based differences in tumor catabolism. Together, these results suggest that high-density, rapidly internalizing antigens subject high-affinity antibodies to greater internalization and degradation, thereby limiting their penetration of tumors. In contrast, lower-affinity antibodies penetrate tumors more effectively when rates of antibody-antigen dissociation are higher than those of antigen internalization. Together, our findings offer insights into how to optimize the ability of therapeutic antibodies to penetrate tumors.
© 2011 AACR.
Figures


Similar articles
-
Increased affinity leads to improved selective tumor delivery of single-chain Fv antibodies.Cancer Res. 1998 Feb 1;58(3):485-90. Cancer Res. 1998. PMID: 9458094
-
High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules.Cancer Res. 2001 Jun 15;61(12):4750-5. Cancer Res. 2001. PMID: 11406547
-
Targeting of bivalent anti-ErbB2 diabody antibody fragments to tumor cells is independent of the intrinsic antibody affinity.Cancer Res. 2000 Nov 15;60(22):6434-40. Cancer Res. 2000. PMID: 11103810
-
Affinity and avidity in antibody-based tumor targeting.Cancer Biother Radiopharm. 2009 Apr;24(2):155-61. doi: 10.1089/cbr.2009.0627. Cancer Biother Radiopharm. 2009. PMID: 19409036 Free PMC article. Review.
-
Targeting HER2 in solid tumors: Unveiling the structure and novel epitopes.Cancer Treat Rev. 2024 Nov;130:102826. doi: 10.1016/j.ctrv.2024.102826. Epub 2024 Sep 4. Cancer Treat Rev. 2024. PMID: 39270365 Review.
Cited by
-
Transient Competitive Inhibition Bypasses the Binding Site Barrier to Improve Tumor Penetration of Trastuzumab and Enhance T-DM1 Efficacy.Cancer Res. 2021 Aug 1;81(15):4145-4154. doi: 10.1158/0008-5472.CAN-20-3822. Epub 2021 Mar 16. Cancer Res. 2021. PMID: 33727230 Free PMC article.
-
Site-specific conjugation allows modulation of click reaction stoichiometry for pretargeted SPECT imaging.MAbs. 2018 Nov-Dec;10(8):1269-1280. doi: 10.1080/19420862.2018.1521132. Epub 2018 Oct 4. MAbs. 2018. PMID: 30199303 Free PMC article.
-
Reducing affinity as a strategy to boost immunomodulatory antibody agonism.Nature. 2023 Feb;614(7948):539-547. doi: 10.1038/s41586-022-05673-2. Epub 2023 Feb 1. Nature. 2023. PMID: 36725933
-
A high-affinity protein binder that blocks the IL-6/STAT3 signaling pathway effectively suppresses non-small cell lung cancer.Mol Ther. 2014 Jul;22(7):1254-1265. doi: 10.1038/mt.2014.59. Epub 2014 Mar 31. Mol Ther. 2014. PMID: 24682171 Free PMC article.
-
Evaluation of a Pretargeting Strategy for Molecular Imaging of the Prostate Stem Cell Antigen with a Single Chain Antibody.Sci Rep. 2018 Feb 28;8(1):3755. doi: 10.1038/s41598-018-22179-y. Sci Rep. 2018. PMID: 29491468 Free PMC article.
References
-
- Fujimori K, Covell DG, Fletcher JE, Weinstein JN. Modeling analysis of the global and microscopic distribution of immunoglobulin G, F(ab')2, and Fab in tumors. Cancer research. 1989;49:5656–63. - PubMed
-
- Fujimori K, Covell DG, Fletcher JE, Weinstein JN. A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J Nucl Med. 1990;31:1191–8. - PubMed
-
- Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer research. 1990;50:814s–9s. - PubMed
-
- Thurber GM, Zajic SC, Wittrup KD. Theoretic criteria for antibody penetration into solid tumors and micrometastases. J Nucl Med. 2007;48:995–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

