Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT
- PMID: 21399631
- PMCID: PMC3066276
- DOI: 10.1038/nn.2781
Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT
Erratum in
- Nat Neurosci. 2011 Dec;14(2):1617
Abstract
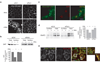
Plasmalemmal neurotransmitter transporters (NTTs) regulate the level of neurotransmitters, such as dopamine (DA) and glutamate, after their release at brain synapses. Stimuli including protein kinase C (PKC) activation can lead to the internalization of some NTTs and a reduction in neurotransmitter clearance capacity. We found that the protein Flotillin-1 (Flot1), also known as Reggie-2, was required for PKC-regulated internalization of members of two different NTT families, the DA transporter (DAT) and the glial glutamate transporter EAAT2, and we identified a conserved serine residue in Flot1 that is essential for transporter internalization. Further analysis revealed that Flot1 was also required to localize DAT within plasma membrane microdomains in stable cell lines, and was essential for amphetamine-induced reverse transport of DA in neurons but not for DA uptake. In sum, our findings provide evidence for a critical role of Flot1-enriched membrane microdomains in PKC-triggered DAT endocytosis and the actions of amphetamine.
Figures



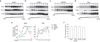

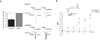
Similar articles
-
Flotillins regulate membrane mobility of the dopamine transporter but are not required for its protein kinase C dependent endocytosis.Traffic. 2013 Jun;14(6):709-24. doi: 10.1111/tra.12059. Epub 2013 Mar 11. Traffic. 2013. PMID: 23418867 Free PMC article.
-
The plasma membrane-associated GTPase Rin interacts with the dopamine transporter and is required for protein kinase C-regulated dopamine transporter trafficking.J Neurosci. 2011 Sep 28;31(39):13758-70. doi: 10.1523/JNEUROSCI.2649-11.2011. J Neurosci. 2011. PMID: 21957239 Free PMC article.
-
Amphetamine-induced decreases in dopamine transporter surface expression are protein kinase C-independent.Neuropharmacology. 2008 Mar;54(3):605-12. doi: 10.1016/j.neuropharm.2007.11.007. Epub 2007 Nov 22. Neuropharmacology. 2008. PMID: 18164041 Free PMC article.
-
Neurotransmitter transporters in schistosomes: structure, function and prospects for drug discovery.Parasitol Int. 2013 Dec;62(6):629-38. doi: 10.1016/j.parint.2013.06.003. Epub 2013 Jun 22. Parasitol Int. 2013. PMID: 23800409 Review.
-
Phosphorylation of the Amino Terminus of the Dopamine Transporter: Regulatory Mechanisms and Implications for Amphetamine Action.Adv Pharmacol. 2018;82:205-234. doi: 10.1016/bs.apha.2017.09.002. Epub 2017 Oct 25. Adv Pharmacol. 2018. PMID: 29413521 Free PMC article. Review.
Cited by
-
Parkinson's disease gene, Synaptojanin1, dysregulates the surface maintenance of the dopamine transporter.NPJ Parkinsons Dis. 2024 Aug 8;10(1):148. doi: 10.1038/s41531-024-00769-0. NPJ Parkinsons Dis. 2024. PMID: 39117637 Free PMC article.
-
Structure of the human dopamine transporter in complex with cocaine.Nature. 2024 Aug;632(8025):678-685. doi: 10.1038/s41586-024-07804-3. Epub 2024 Aug 7. Nature. 2024. PMID: 39112703
-
Enhanced therapeutic potential of Flotillins-modified MenSCs by improve the survival, proliferation and migration.Mol Biol Rep. 2024 May 25;51(1):680. doi: 10.1007/s11033-024-09624-0. Mol Biol Rep. 2024. PMID: 38796595
-
Dopamine transporter membrane mobility is bidirectionally regulated by phosphorylation and palmitoylation.Curr Res Physiol. 2023 Sep 29;6:100106. doi: 10.1016/j.crphys.2023.100106. eCollection 2023. Curr Res Physiol. 2023. PMID: 38107792 Free PMC article. Review.
-
The roles of FLOT1 in human diseases (Review).Mol Med Rep. 2023 Nov;28(5):212. doi: 10.3892/mmr.2023.13099. Epub 2023 Sep 29. Mol Med Rep. 2023. PMID: 37772385 Free PMC article. Review.
References
-
- Melikian HE. Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacol Ther. 2004;104:17–27. - PubMed
-
- Kahlig KM, Javitch JA, Galli A. Amphetamine regulation of dopamine transport. Combined measurements of transporter currents and transporter imaging support the endocytosis of an active carrier. J Biol Chem. 2004;279:8966–8975. - PubMed
-
- Gonzalez MI, Susarla BT, Robinson MB. Evidence that protein kinase Calpha interacts with and regulates the glial glutamate transporter GLT-1. J Neurochem. 2005;94:1180–1188. - PubMed
-
- Kalandadze A, Wu Y, Robinson MB. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J Biol Chem. 2002;277:45741–45750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS050199-05/NS/NINDS NIH HHS/United States
- DA13975/DA/NIDA NIH HHS/United States
- R01 NS050199/NS/NINDS NIH HHS/United States
- R01 DA013975/DA/NIDA NIH HHS/United States
- R01 DA013975-11/DA/NIDA NIH HHS/United States
- K05 DA022413-05/DA/NIDA NIH HHS/United States
- R56 DA013975/DA/NIDA NIH HHS/United States
- K05 DA022413/DA/NIDA NIH HHS/United States
- R01 DA013147/DA/NIDA NIH HHS/United States
- P01 DA012408-12/DA/NIDA NIH HHS/United States
- P01 DA012408/DA/NIDA NIH HHS/United States
- DA13147/DA/NIDA NIH HHS/United States
- P01 DA12408/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

