Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells
- PMID: 21399623
- PMCID: PMC3077249
- DOI: 10.1038/embor.2011.28
Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells
Abstract
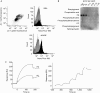
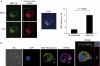
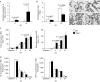
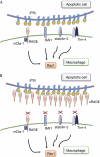
Clearance of apoptotic cells is necessary for tissue development, homeostasis and resolution of inflammation. The uptake of apoptotic cells is initiated by an 'eat-me' signal, such as phosphatidylserine, on the cell surface and phagocytes recognize the signal by using specific receptors. In this study, we show that the soluble form of the receptor for advanced glycation end products (RAGE) binds to phosphatidylserine as well as to the apoptotic thymocytes. RAGE-deficient (Rage(-/-)) alveolar macrophages showed impaired phagocytosis of apoptotic thymocytes and defective clearance of apoptotic neutrophils in Rage(-/-) mice. Our results indicate that RAGE functions as a phosphatidylserine receptor and assists in the clearance of apoptotic cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Comment in
-
Phosphatidylserine receptors: what is the new RAGE?EMBO Rep. 2011 Apr;12(4):287-8. doi: 10.1038/embor.2011.41. Epub 2011 Mar 11. EMBO Rep. 2011. PMID: 21399618 Free PMC article. No abstract available.
Similar articles
-
Participation of the receptor for advanced glycation end products in efferocytosis.J Immunol. 2011 Jun 1;186(11):6191-8. doi: 10.4049/jimmunol.1004134. Epub 2011 Apr 18. J Immunol. 2011. PMID: 21502377 Free PMC article.
-
The role of phosphatidylserine recognition receptors in multiple biological functions.Cell Mol Biol Lett. 2020 Mar 26;25:23. doi: 10.1186/s11658-020-00214-z. eCollection 2020. Cell Mol Biol Lett. 2020. PMID: 32226456 Free PMC article. Review.
-
An Apoptotic 'Eat Me' Signal: Phosphatidylserine Exposure.Trends Cell Biol. 2015 Nov;25(11):639-650. doi: 10.1016/j.tcb.2015.08.003. Epub 2015 Oct 1. Trends Cell Biol. 2015. PMID: 26437594 Review.
-
Draper-mediated and phosphatidylserine-independent phagocytosis of apoptotic cells by Drosophila hemocytes/macrophages.J Biol Chem. 2004 Nov 12;279(46):48466-76. doi: 10.1074/jbc.M408597200. Epub 2004 Sep 1. J Biol Chem. 2004. PMID: 15342648
-
Phosphatidylserine recognition and induction of apoptotic cell clearance by Drosophila engulfment receptor Draper.J Biochem. 2013 May;153(5):483-91. doi: 10.1093/jb/mvt014. Epub 2013 Feb 18. J Biochem. 2013. PMID: 23420848
Cited by
-
Involvement of Receptor for Advanced Glycation Endproducts in Hypertensive Disorders of Pregnancy.Int J Mol Sci. 2019 Nov 1;20(21):5462. doi: 10.3390/ijms20215462. Int J Mol Sci. 2019. PMID: 31683992 Free PMC article.
-
The RAGE/DIAPH1 Signaling Axis & Implications for the Pathogenesis of Diabetic Complications.Int J Mol Sci. 2022 Apr 21;23(9):4579. doi: 10.3390/ijms23094579. Int J Mol Sci. 2022. PMID: 35562970 Free PMC article. Review.
-
Phosphatidylserine, inflammation, and central nervous system diseases.Front Aging Neurosci. 2022 Aug 3;14:975176. doi: 10.3389/fnagi.2022.975176. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35992593 Free PMC article. Review.
-
Contribution of Defective PS Recognition and Efferocytosis to Chronic Inflammation and Autoimmunity.Front Immunol. 2014 Nov 10;5:566. doi: 10.3389/fimmu.2014.00566. eCollection 2014. Front Immunol. 2014. PMID: 25426118 Free PMC article. Review.
-
Efferocytosis: An Emerging Therapeutic Strategy for Type 2 Diabetes Mellitus and Diabetes Complications.J Inflamm Res. 2023 Jul 7;16:2801-2815. doi: 10.2147/JIR.S418334. eCollection 2023. J Inflamm Res. 2023. PMID: 37440994 Free PMC article. Review.
References
-
- Albert ML, Kim JI, Birge RB (2000) αvβ5 integrin recruits the CrkII–Dock180–rac1 complex for phagocytosis of apoptotic cells. Nat Cell Biol 2: 899–905 - PubMed
-
- Cox G, Crossley J, Xing Z (1995) Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol 12: 232–237 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

