Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow
- PMID: 21381023
- PMCID: PMC3117029
- DOI: 10.1002/jcp.22715
Mechanisms of ATP release by human trabecular meshwork cells, the enabling step in purinergic regulation of aqueous humor outflow
Abstract
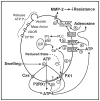
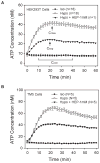
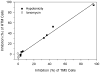
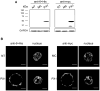
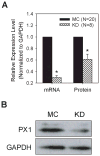
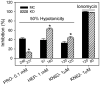
Our guiding hypothesis is that ecto-enzymatic conversion of extracellular ATP to adenosine activates A(1) adenosine receptors, reducing resistance to aqueous humor outflow and intraocular pressure. The initial step in this purinergic regulation is ATP release from outflow-pathway cells by mechanisms unknown. We measured similar ATP release from human explant-derived primary trabecular meshwork (TM) cells (HTM) and a human TM cell line (TM5). Responses to 21 inhibitors indicated that pannexin-1 (PX1) and connexin (Cx) hemichannels and P2X(7) receptors (P2RX(7) ) were comparably important in modulating ATP release induced by hypotonic swelling, whereas vesicular release was insignificant. Consistent with prior studies of PX1 activity in certain other cells, ATP release was lowered by the reducing agent dithiothreitol. Overexpressing PX1 in HEK293T cells promoted, while partial knockdown (KD) in both HEK293T and TM5 cells inhibited hypotonicity-activated ATP release. Additionally, KD reduced the pharmacologically defined contribution of PX1 and enhanced those of Cx and P2RX(7) . ATP release was also triggered by raising intracellular Ca(2+) activity with ionomycin after a prolonged lag time and was unaffected by the PX1 blocker probenecid, but nearly abolished by P2RX(7) antagonists. We conclude that swelling-stimulated ATP release from human TM cells is physiologically mediated by PX1 and Cx hemichannels and P2X(7) receptors, but not by vesicular release. PX1 appears not to be stimulated by intracellular Ca(2+) in TM cells, but can be modulated by oxidation-reduction state. The P2RX(7) -dependent component of swelling-activated release may be mediated by PX1 hemichannels or reflect apoptotic magnification of ATP release, either through itself and/or hemichannels.
Copyright © 2011 Wiley Periodicals, Inc.
Figures

Similar articles
-
Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow.Am J Physiol Cell Physiol. 2010 Dec;299(6):C1308-17. doi: 10.1152/ajpcell.00333.2010. Epub 2010 Oct 6. Am J Physiol Cell Physiol. 2010. PMID: 20926783 Free PMC article.
-
Cytoskeletal dependence of adenosine triphosphate release by human trabecular meshwork cells.Invest Ophthalmol Vis Sci. 2011 Oct 10;52(11):7996-8005. doi: 10.1167/iovs.11-8170. Invest Ophthalmol Vis Sci. 2011. PMID: 21896846 Free PMC article.
-
Mechanisms of ATP release, the enabling step in purinergic dynamics.Cell Physiol Biochem. 2011;28(6):1135-44. doi: 10.1159/000335865. Epub 2011 Dec 16. Cell Physiol Biochem. 2011. PMID: 22179002 Free PMC article. Review.
-
Effects of cardiotonic steroids on trabecular meshwork cells: search for mediator of ouabain-enhanced outflow facility.Exp Eye Res. 2012 Mar;96(1):4-12. doi: 10.1016/j.exer.2012.01.009. Epub 2012 Jan 24. Exp Eye Res. 2012. PMID: 22300616 Free PMC article.
-
Extracellular matrix in the trabecular meshwork: intraocular pressure regulation and dysregulation in glaucoma.Exp Eye Res. 2015 Apr;133:112-25. doi: 10.1016/j.exer.2014.07.014. Exp Eye Res. 2015. PMID: 25819459 Free PMC article. Review.
Cited by
-
P2X₇ receptor activation may be involved in neuronal loss in the retinal ganglion cell layer after acute elevation of intraocular pressure in rats.Mol Vis. 2013 Sep 28;19:2080-91. eCollection 2013. Mol Vis. 2013. PMID: 24146541 Free PMC article.
-
Molecular pathways of pannexin1-mediated neurotoxicity.Front Physiol. 2014 Feb 11;5:23. doi: 10.3389/fphys.2014.00023. eCollection 2014. Front Physiol. 2014. PMID: 24575045 Free PMC article. Review.
-
Targeting Schlemm's Canal in the Medical Therapy of Glaucoma: Current and Future Considerations.Adv Ther. 2017 May;34(5):1049-1069. doi: 10.1007/s12325-017-0513-z. Epub 2017 Mar 27. Adv Ther. 2017. PMID: 28349508 Free PMC article. Review.
-
Therapeutic Drugs and Devices for Tackling Ocular Hypertension and Glaucoma, and Need for Neuroprotection and Cytoprotective Therapies.Front Pharmacol. 2021 Sep 17;12:729249. doi: 10.3389/fphar.2021.729249. eCollection 2021. Front Pharmacol. 2021. PMID: 34603044 Free PMC article. Review.
-
Targeting the NLRP3 Inflammasome in Glaucoma.Biomolecules. 2021 Aug 19;11(8):1239. doi: 10.3390/biom11081239. Biomolecules. 2021. PMID: 34439904 Free PMC article. Review.
References
-
- Al-Aswad LA, Gong H, Lee D, O’Donnell ME, Brandt JD, Ryan WJ, Schroeder A, Erickson KA. Effects of Na-K-2Cl cotransport regulators on outflow facility in calf and human eyes in vitro. Invest Ophthalmol Vis Sci. 1999;40(8):1695–1701. - PubMed
-
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004a;572(1–3):65–68. - PubMed
-
- Bao L, Sachs F, Dahl G. Connexins are mechanosensitive. Am J Physiol Cell Physiol. 2004b;287(5):C1389–1395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

