Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium
- PMID: 21368224
- PMCID: PMC6016742
- DOI: 10.4049/jimmunol.1002961
Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium
Abstract
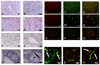
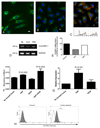
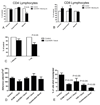
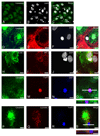
The common lymphatic endothelial and vascular endothelial receptor (CLEVER-1; also known as FEEL-1 and stabilin-1) is a recycling and intracellular trafficking receptor with multifunctional properties. In this study, we demonstrate increased endothelial expression of CLEVER-1/stabilin-1 at sites of leukocyte recruitment to the inflamed human liver including sinusoids, septal vessels, and lymphoid follicles in inflammatory liver disease and tumor-associated vessels in hepatocellular carcinoma. We used primary cultures of human hepatic sinusoidal endothelial cells (HSEC) to demonstrate that CLEVER-1/stabilin-1 expression is enhanced by hepatocyte growth factor but not by classical proinflammatory cytokines. We then showed that CLEVER-1/stabilin-1 supports T cell transendothelial migration across HSEC under conditions of flow with strong preferential activity for CD4 FoxP3(+) regulatory T cells (Tregs). CLEVER-1/stabilin-1 inhibition reduced Treg transendothelial migration by 40% and when combined with blockade of ICAM-1 and vascular adhesion protein-1 (VAP-1) reduced it by >80%. Confocal microscopy demonstrated that 60% of transmigrating Tregs underwent transcellular migration through HSEC via ICAM-1- and VAP-1-rich transcellular pores in close association with CLEVER-1/stabilin-1. Thus, CLEVER-1/stabilin-1 and VAP-1 may provide an organ-specific signal for Treg recruitment to the inflamed liver and to hepatocellular carcinoma.
Figures

Similar articles
-
CLEVER-1 mediates lymphocyte transmigration through vascular and lymphatic endothelium.Blood. 2004 Dec 15;104(13):3849-57. doi: 10.1182/blood-2004-01-0222. Epub 2004 Aug 5. Blood. 2004. PMID: 15297319
-
Stabilin-1/CLEVER-1, a type 2 macrophage marker, is an adhesion and scavenging molecule on human placental macrophages.Eur J Immunol. 2011 Jul;41(7):2052-63. doi: 10.1002/eji.201041376. Epub 2011 Jun 1. Eur J Immunol. 2011. PMID: 21480214
-
Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver.J Immunol. 2010 Mar 15;184(6):2886-98. doi: 10.4049/jimmunol.0901216. Epub 2010 Feb 17. J Immunol. 2010. PMID: 20164417
-
Stabilin-1, a homeostatic scavenger receptor with multiple functions.J Cell Mol Med. 2006 Jul-Sep;10(3):635-49. doi: 10.1111/j.1582-4934.2006.tb00425.x. J Cell Mol Med. 2006. PMID: 16989725 Free PMC article. Review.
-
Multifunctional receptor stabilin-1 in homeostasis and disease.ScientificWorldJournal. 2010 Oct 12;10:2039-53. doi: 10.1100/tsw.2010.189. ScientificWorldJournal. 2010. PMID: 20953554 Free PMC article. Review.
Cited by
-
Prominent Receptors of Liver Sinusoidal Endothelial Cells in Liver Homeostasis and Disease.Front Physiol. 2020 Jul 21;11:873. doi: 10.3389/fphys.2020.00873. eCollection 2020. Front Physiol. 2020. PMID: 32848838 Free PMC article. Review.
-
Targeting the tumor vasculature to enhance T cell activity.Curr Opin Immunol. 2015 Apr;33:55-63. doi: 10.1016/j.coi.2015.01.011. Epub 2015 Feb 6. Curr Opin Immunol. 2015. PMID: 25665467 Free PMC article. Review.
-
Stromal Cells Underlining the Paths From Autoimmunity, Inflammation to Cancer With Roles Beyond Structural and Nutritional Support.Front Cell Dev Biol. 2021 May 25;9:658984. doi: 10.3389/fcell.2021.658984. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34113615 Free PMC article. Review.
-
Nonclinical Characterization of Bexmarilimab, a Clever-1-Targeting Antibody for Supporting Immune Defense Against Cancers.Mol Cancer Ther. 2022 Jul 5;21(7):1207-1218. doi: 10.1158/1535-7163.MCT-21-0840. Mol Cancer Ther. 2022. PMID: 35500016 Free PMC article. Clinical Trial.
-
The Crosstalk Between Liver Sinusoidal Endothelial Cells and Hepatic Microenvironment in NASH Related Liver Fibrosis.Front Immunol. 2022 Jun 28;13:936196. doi: 10.3389/fimmu.2022.936196. eCollection 2022. Front Immunol. 2022. PMID: 35837401 Free PMC article. Review.
References
-
- Xu XD, Ueta H, Zhou S, Shi C, Koga D, Ushiki T, Matsuno K. Trafficking of recirculating lymphocytes in the rat liver: rapid transmigration into the portal area and then to the hepatic lymph. Liver Int. 2008;28:319–330. - PubMed
-
- Lalor PF, Shields P, Grant A, Adams DH. Recruitment of lymphocytes to the human liver. Immunol Cell Biol. 2002;80:52–64. - PubMed
-
- Adams DH, Hubscher SG, Fisher NC, Williams A, Robinson M. Expression of E-selectin and E-selectin ligands in human liver inflammation. Hepatology. 1996;24:533–538. - PubMed
-
- Steinhoff G, Behrend M, Schrader B, Duijvestijn AM, Wonigeit K. Expression patterns of leukocyte adhesion ligand molecules on human liver endothelia - lack of ELAM-1 and CD62 inducibility on sinusoidal endothelia and distinct distribution of VCAM-1, ICAM-1, ICAM-2 and LFA-3. American Journal of Pathology. 1993;142:481–488. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

