Mutant IDH1 confers an in vivo growth in a melanoma cell line with BRAF mutation
- PMID: 21356389
- PMCID: PMC3069821
- DOI: 10.1016/j.ajpath.2010.12.011
Mutant IDH1 confers an in vivo growth in a melanoma cell line with BRAF mutation
Abstract
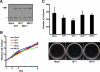
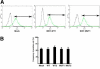
Melanoma is the most deadly tumor of the skin, and systemic therapies for the advanced stage are still limited. Recent genetic analyses have revealed the molecular diversity of melanoma and potential therapeutic targets. By screening a cohort of 142 primary nonepithelial tumors, we discovered that about 10% of melanoma cases (4/39) harbored an IDH1 or IDH2 mutation. These mutations were found to coexist with BRAF or KIT mutation, and all IDH1 mutations were detected in metastatic lesions. BRAF-mutated melanoma cells, additionally expressing the cancer-related IDH1 mutant, acquired increased colony-forming and in vivo growth activities and showed enhanced activation of the MAPK and STAT3 pathways. Genome-wide gene expression profiling demonstrated that mutant IDH1 affected the expression of a set of genes. Especially, it caused the induction of growth-related transcriptional regulators (Jun, N-myc, Atf3) and the reduction of Rassf1 and two dehydrogenase genes (Dhrs1 and Adh5), which may be involved in the carcinogenesis of IDH1-mutated tumors. Our analyses demonstrate that IDH1 mutation works with other oncogenic mutations and could contribute to the metastasis in melanoma.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


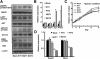
Similar articles
-
The BRAF(V600E) causes widespread alterations in gene methylation in the genome of melanoma cells.Cell Cycle. 2012 Jan 15;11(2):286-95. doi: 10.4161/cc.11.2.18707. Epub 2012 Jan 15. Cell Cycle. 2012. PMID: 22189819 Free PMC article.
-
Novel somatic mutations to PI3K pathway genes in metastatic melanoma.PLoS One. 2012;7(8):e43369. doi: 10.1371/journal.pone.0043369. Epub 2012 Aug 17. PLoS One. 2012. PMID: 22912864 Free PMC article.
-
ROS production induced by BRAF inhibitor treatment rewires metabolic processes affecting cell growth of melanoma cells.Mol Cancer. 2017 Jun 8;16(1):102. doi: 10.1186/s12943-017-0667-y. Mol Cancer. 2017. PMID: 28595656 Free PMC article.
-
Analysis of IDH1-R132 mutation, BRAF V600 mutation and KIAA1549-BRAF fusion transcript status in central nervous system tumors supports pediatric tumor classification.J Cancer Res Clin Oncol. 2016 Jan;142(1):89-100. doi: 10.1007/s00432-015-2006-2. Epub 2015 Jun 27. J Cancer Res Clin Oncol. 2016. PMID: 26115961
-
Transcriptional regulators and alterations that drive melanoma initiation and progression.Oncogene. 2020 Nov;39(48):7093-7105. doi: 10.1038/s41388-020-01490-x. Epub 2020 Oct 6. Oncogene. 2020. PMID: 33024276 Free PMC article. Review.
Cited by
-
Melanoma genomics - will we go beyond BRAF in clinics?J Cancer Res Clin Oncol. 2024 Sep 28;150(9):433. doi: 10.1007/s00432-024-05957-2. J Cancer Res Clin Oncol. 2024. PMID: 39340537 Free PMC article. Review.
-
Transcriptome-Wide Association Study Reveals New Molecular Interactions Associated with Melanoma Pathogenesis.Cancers (Basel). 2024 Jul 11;16(14):2517. doi: 10.3390/cancers16142517. Cancers (Basel). 2024. PMID: 39061157 Free PMC article.
-
Are IDH1 R132 Mutations Associated With Poor Prognosis in Patients With Chondrosarcoma of the Bone?Clin Orthop Relat Res. 2024 Jan 3;482(6):947-56. doi: 10.1097/CORR.0000000000002960. Online ahead of print. Clin Orthop Relat Res. 2024. PMID: 38170705
-
Isocitrate Dehydrogenase 1 Mutation and Ivosidenib in Patients With Acute Myeloid Leukemia: A Comprehensive Review.Cureus. 2023 Sep 6;15(9):e44802. doi: 10.7759/cureus.44802. eCollection 2023 Sep. Cureus. 2023. PMID: 37692182 Free PMC article. Review.
-
Characterization of purinergic signaling in tumor-infiltrating lymphocytes from lower- and high-grade gliomas.Purinergic Signal. 2024 Feb;20(1):47-64. doi: 10.1007/s11302-023-09931-4. Epub 2023 Mar 24. Purinergic Signal. 2024. PMID: 36964277 Free PMC article.
References
-
- Tsao H., Atkins M.B., Sober A.J. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. - PubMed
-
- Verma S., Quirt I., McCready D., Bak K., Charette M., Iscoe N. Systematic review of systemic adjuvant therapy for patients at high risk for recurrent melanoma. Cancer. 2006;106:1431–1442. - PubMed
-
- Kuphal S., Bosserhoff A. Recent progress in understanding the pathology of malignant melanoma. J Pathol. 2009;219:400–409. - PubMed
-
- Curtin J.A., Fridlyand J., Kageshita T., Patel H.N., Busam K.J., Kutzner H., Cho K.H., Aiba S., Bröcker E.B., LeBoit P.E., Pinkel D., Bastian B.C. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. - PubMed
-
- Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., Davis N., Dicks E., Ewing R., Floyd Y., Gray K., Hall S., Hawes R., Hughes J., Kosmidou V., Menzies A., Mould C., Parker A., Stevens C., Watt S., Hooper S., Wilson R., Jayatilake H., Gusterson B.A., Cooper C., Shipley J., Hargrave D., Pritchard-Jones K., Maitland N., Chenevix-Trench G., Riggins G.J., Bigner D.D., Palmieri G., Cossu A., Flanagan A., Nicholson A., Ho J.W., Leung S.Y., Yuen S.T., Weber B.L., Seigler H.F., Darrow T.L., Paterson H., Marais R., Marshall C.J., Wooster R., Stratton M.R., Futreal P.A. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

