Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections
- PMID: 21353708
- PMCID: PMC3094911
- DOI: 10.1016/j.jri.2011.01.005
Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections
Abstract
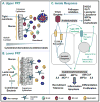
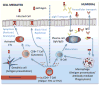
This review examines the multiple levels of pre-existing immunity in the upper and lower female reproductive tract. In addition, we highlight the need for further research of innate and adaptive immune protection of mucosal surfaces in the female reproductive tract. Innate mechanisms include the mucus lining, a tight epithelial barrier and the secretion of antimicrobial peptides and cytokines by epithelial and innate immune cells. Stimulation of the innate immune system also serves to bridge the adaptive arm resulting in the generation of pathogen-specific humoral and cell-mediated immunity. Less understood are the multiple components that act in a coordinated way to provide a network of ongoing protection. Innate and adaptive immunity in the human female reproductive tract are influenced by the stage of menstrual cycle and are directly regulated by the sex steroid hormones, progesterone and estradiol. Furthermore, the effect of hormones on immunity is mediated both directly on immune and epithelial cells and indirectly by stimulating growth factor secretion from stromal cells. The goal of this review is to focus on the diverse aspects of the innate and adaptive immune systems that contribute to a unique network of protection throughout the female reproductive tract.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures
Similar articles
-
Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens.Am J Reprod Immunol. 2010 Jun;63(6):544-65. doi: 10.1111/j.1600-0897.2010.00842.x. Epub 2010 Mar 29. Am J Reprod Immunol. 2010. PMID: 20367623 Free PMC article.
-
Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections.Am J Reprod Immunol. 2011 Mar;65(3):196-211. doi: 10.1111/j.1600-0897.2011.00970.x. Am J Reprod Immunol. 2011. PMID: 21294805 Free PMC article. Review.
-
The impact of aging on innate and adaptive immunity in the human female genital tract.Aging Cell. 2021 May;20(5):e13361. doi: 10.1111/acel.13361. Epub 2021 May 5. Aging Cell. 2021. PMID: 33951269 Free PMC article. Review.
-
The role of sex hormones in immune protection of the female reproductive tract.Nat Rev Immunol. 2015 Apr;15(4):217-30. doi: 10.1038/nri3819. Epub 2015 Mar 6. Nat Rev Immunol. 2015. PMID: 25743222 Free PMC article. Review.
-
Innate and acquired immunity in the human penile urethra.J Reprod Immunol. 2011 Mar;88(2):219-27. doi: 10.1016/j.jri.2011.01.006. Epub 2011 Feb 24. J Reprod Immunol. 2011. PMID: 21353311 Free PMC article. Review.
Cited by
-
Antiviral lectin Q-Griffithsin suppresses fungal infection in murine models of vaginal candidiasis.Front Cell Infect Microbiol. 2022 Oct 18;12:976033. doi: 10.3389/fcimb.2022.976033. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36329822 Free PMC article.
-
Pathogen recognition in the human female reproductive tract: expression of intracellular cytosolic sensors NOD1, NOD2, RIG-1, and MDA5 and response to HIV-1 and Neisseria gonorrhea.Am J Reprod Immunol. 2013 Jan;69(1):41-51. doi: 10.1111/aji.12019. Epub 2012 Sep 17. Am J Reprod Immunol. 2013. PMID: 22984986 Free PMC article.
-
BLT humanized mice as model to study HIV vaginal transmission.J Infect Dis. 2013 Nov;208 Suppl 2(Suppl 2):S131-6. doi: 10.1093/infdis/jit318. J Infect Dis. 2013. PMID: 24151319 Free PMC article. Review.
-
The role of sex hormones and the tissue environment in immune protection against HIV in the female reproductive tract.Am J Reprod Immunol. 2014 Aug;72(2):171-81. doi: 10.1111/aji.12235. Epub 2014 Mar 24. Am J Reprod Immunol. 2014. PMID: 24661500 Free PMC article. Review.
-
Cervico-vaginal immunoglobulin G levels increase post-ovulation independently of neutrophils.PLoS One. 2014 Dec 5;9(12):e114824. doi: 10.1371/journal.pone.0114824. eCollection 2014. PLoS One. 2014. PMID: 25479383 Free PMC article.
References
-
- Ahmed N, et al. Suppression of human immunodeficiency virus type 1 replication in macrophages by commensal bacteria preferentially stimulating Toll-like receptor 4. J Gen Virol. 2010;91:2804–2813. - PubMed
-
- Arruvito L, et al. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol. 2007;178:2572–2578. - PubMed
-
- Bazer FW, et al. Interferons and uterine receptivity. Semin Reprod Med. 2009;27:90–102. - PubMed
-
- Bélec L, Pillot J. Raped women and HIV infection. J Forensic Sci. 1995;40:925–926. - PubMed
-
- Black CA, et al. Vaginal mucosa serves as an inductive site for tolerance. J Immunol. 2000;165:5077–5083. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

