Incomplete activation of peripheral blood dendritic cells during healthy human pregnancy
- PMID: 21352205
- PMCID: PMC3087910
- DOI: 10.1111/j.1365-2249.2011.04330.x
Incomplete activation of peripheral blood dendritic cells during healthy human pregnancy
Abstract
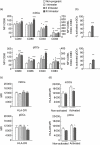
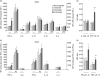
Successful pregnancy relies on the adaptation of immune responses that allow the fetus to grow and develop in the uterus despite being recognized by maternal immune cells. Dendritic cells (DCs) are central to the control of immune tolerance, and their state of activation at the maternal-decidual interface is critical to the feto-maternal immunological equilibrium. So far, the involvement of circulating DCs has been investigated poorly. Therefore, in this study we investigated whether, during healthy human pregnancy, peripheral blood DCs (PBDCs) undergo changes that may be relevant to the adaptation of maternal immune responses that allow fetal tolerance. In a cross-sectional study, we analysed PBDCs by six-colour flow cytometry on whole blood samples from 47 women during healthy pregnancy progression and 24 non-pregnant controls. We demonstrated that both myeloid and plasmacytoid PBDCs undergo a state of incomplete activation, more evident in the third trimester, characterized by increased expression of co-stimulatory molecules and cytokine production but lacking human leucocyte antigen (HLA)-DR up-regulation. To investigate the contribution of soluble circulating factors to this phenomenon, we also performed culture experiments showing that sera from pregnant women added to control DCs conditioned a similar incomplete activation that was associated with reduced DC allostimulatory capacity, supporting the in vivo relevance of our findings. We also obtained evidence that the glycoprotein hormone activin-A may contribute to DC incomplete activation. We suggest that the changes of PBDCs occurring during late pregnancy may aid the comprehension of the immune mechanisms operated by the maternal immune system to maintain fetal tolerance.
© 2011 The Authors; Clinical and Experimental Immunology © 2011 British Society for Immunology.
Figures



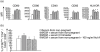
Similar articles
-
Lack of activation of peripheral blood dendritic cells in human pregnancies complicated by intrauterine growth restriction.Placenta. 2013 Jan;34(1):35-41. doi: 10.1016/j.placenta.2012.10.016. Epub 2012 Nov 22. Placenta. 2013. PMID: 23182380
-
The glycoprotein-hormones activin A and inhibin A interfere with dendritic cell maturation.Reprod Biol Endocrinol. 2008 May 6;6:17. doi: 10.1186/1477-7827-6-17. Reprod Biol Endocrinol. 2008. PMID: 18460206 Free PMC article.
-
Immunosuppressive effect of pregnant mouse serum on allostimulatory activity of dendritic cells.J Reprod Immunol. 2007 Aug;75(1):23-31. doi: 10.1016/j.jri.2007.02.006. Epub 2007 Apr 16. J Reprod Immunol. 2007. PMID: 17434209
-
Role of dendritic cells in the regulation of maternal immune responses to the fetus during mammalian gestation.Immunol Invest. 2008;37(5):499-533. doi: 10.1080/08820130802191334. Immunol Invest. 2008. PMID: 18716936 Review.
-
Dendritic Cells and the Establishment of Fetomaternal Tolerance for Successful Human Pregnancy.Arch Immunol Ther Exp (Warsz). 2024 May 23;72(1). doi: 10.2478/aite-2024-0010. eCollection 2024 Jan 1. Arch Immunol Ther Exp (Warsz). 2024. PMID: 38782369 Review.
Cited by
-
Peripheral blood cell signatures of Plasmodium falciparum infection during pregnancy.PLoS One. 2012;7(12):e49621. doi: 10.1371/journal.pone.0049621. Epub 2012 Dec 11. PLoS One. 2012. PMID: 23239967 Free PMC article.
-
Mechanisms of Key Innate Immune Cells in Early- and Late-Onset Preeclampsia.Front Immunol. 2020 Aug 18;11:1864. doi: 10.3389/fimmu.2020.01864. eCollection 2020. Front Immunol. 2020. PMID: 33013837 Free PMC article. Review.
-
The first trimester gravid serum regulates procalcitonin expression in human macrophages skewing their phenotype in vitro.Mediators Inflamm. 2014;2014:248963. doi: 10.1155/2014/248963. Epub 2014 Mar 5. Mediators Inflamm. 2014. PMID: 24733960 Free PMC article.
-
Liaison between natural killer cells and dendritic cells in human gestation.Cell Mol Immunol. 2014 Sep;11(5):449-55. doi: 10.1038/cmi.2014.36. Epub 2014 Jun 23. Cell Mol Immunol. 2014. PMID: 24954224 Free PMC article. Review.
-
Immunobiology of Acute Chorioamnionitis.Front Immunol. 2020 Apr 16;11:649. doi: 10.3389/fimmu.2020.00649. eCollection 2020. Front Immunol. 2020. PMID: 32373122 Free PMC article. Review.
References
-
- Veenstra Van Nieuwenhoven AL, Heineman MJ, Faas MM. The immunology of successful pregnancy. Hum Reprod. 2003;9:347–57. - PubMed
-
- Von Rango U. Fetal tolerance in human pregnancy – a crucial balance between acceptance and limitation of trophoblast invasion. Immunol Lett. 2008;115:21–32. - PubMed
-
- Rossi M, Young JW. Human dendritic cells: potent antigen-processing cells at the crossroads of innate and adaptive immunity. J Immunol. 2005;175:1373–81. - PubMed
-
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. - PubMed
-
- Della Bella S, Clerici M, Villa ML. Disarming dendritic cells: a tumor strategy to escape from immune control. Expert Rev Clin Immunol. 2007;3:411–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

