Classification of mouse sperm motility patterns using an automated multiclass support vector machines model
- PMID: 21349820
- PMCID: PMC3099585
- DOI: 10.1095/biolreprod.110.088989
Classification of mouse sperm motility patterns using an automated multiclass support vector machines model
Abstract
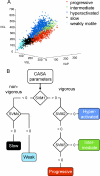
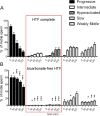
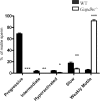
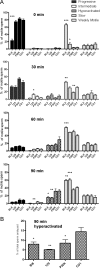
Vigorous sperm motility, including the transition from progressive to hyperactivated motility that occurs in the female reproductive tract, is required for normal fertilization in mammals. We developed an automated, quantitative method that objectively classifies five distinct motility patterns of mouse sperm using Support Vector Machines (SVM), a common method in supervised machine learning. This multiclass SVM model is based on more than 2000 sperm tracks that were captured by computer-assisted sperm analysis (CASA) during in vitro capacitation and visually classified as progressive, intermediate, hyperactivated, slow, or weakly motile. Parameters associated with the classified tracks were incorporated into established SVM algorithms to generate a series of equations. These equations were integrated into a binary decision tree that sequentially sorts uncharacterized tracks into distinct categories. The first equation sorts CASA tracks into vigorous and nonvigorous categories. Additional equations classify vigorous tracks as progressive, intermediate, or hyperactivated and nonvigorous tracks as slow or weakly motile. Our CASAnova software uses these SVM equations to classify individual sperm motility patterns automatically. Comparisons of motility profiles from sperm incubated with and without bicarbonate confirmed the ability of the model to distinguish hyperactivated patterns of motility that develop during in vitro capacitation. The model accurately classifies motility profiles of sperm from a mutant mouse model with severe motility defects. Application of the model to sperm from multiple inbred strains reveals strain-dependent differences in sperm motility profiles. CASAnova provides a rapid and reproducible platform for quantitative comparisons of motility in large, heterogeneous populations of mouse sperm.
Figures

Similar articles
-
CASAnova: a multiclass support vector machine model for the classification of human sperm motility patterns.Biol Reprod. 2017 Nov 1;97(5):698-708. doi: 10.1093/biolre/iox120. Biol Reprod. 2017. PMID: 29036474 Free PMC article.
-
Taking advantage of the use of supervised learning methods for characterization of sperm population structure related with freezability in the Iberian red deer.Theriogenology. 2012 May;77(8):1661-72. doi: 10.1016/j.theriogenology.2011.12.011. Epub 2012 Feb 14. Theriogenology. 2012. PMID: 22341709
-
Development of computer-directed methods for the identification of hyperactivated motion using motion patterns developed by rabbit sperm during incubation under capacitation conditions.J Androl. 1994 Jul-Aug;15(4):362-77. J Androl. 1994. PMID: 7982805
-
Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish.Comp Biochem Physiol C Toxicol Pharmacol. 2001 Dec;130(4):425-33. doi: 10.1016/s1532-0456(01)00270-8. Comp Biochem Physiol C Toxicol Pharmacol. 2001. PMID: 11738630 Review.
-
CASA-Mot in mammals: an update.Reprod Fertil Dev. 2018 Jun;30(6):799-809. doi: 10.1071/RD17432. Reprod Fertil Dev. 2018. PMID: 29514734 Review.
Cited by
-
Metabolic substrates exhibit differential effects on functional parameters of mouse sperm capacitation.Biol Reprod. 2012 Sep 28;87(3):75. doi: 10.1095/biolreprod.112.102673. Print 2012 Sep. Biol Reprod. 2012. PMID: 22837480 Free PMC article.
-
Effect of High Viscosity on Energy Metabolism and Kinematics of Spermatozoa from Three Mouse Species Incubated under Capacitating Conditions.Int J Mol Sci. 2022 Dec 3;23(23):15247. doi: 10.3390/ijms232315247. Int J Mol Sci. 2022. PMID: 36499575 Free PMC article.
-
Sperm membrane proteins DCST1 and DCST2 are required for sperm-egg interaction in mice and fish.Commun Biol. 2022 Apr 7;5(1):332. doi: 10.1038/s42003-022-03289-w. Commun Biol. 2022. PMID: 35393517 Free PMC article.
-
Human Semenogelin 1 Promotes Sperm Survival in the Mouse Female Reproductive Tract.Int J Mol Sci. 2020 May 31;21(11):3961. doi: 10.3390/ijms21113961. Int J Mol Sci. 2020. PMID: 32486486 Free PMC article.
-
Aurora A Kinase (AURKA) is required for male germline maintenance and regulates sperm motility in the mouse.Biol Reprod. 2021 Dec 20;105(6):1603-1616. doi: 10.1093/biolre/ioab168. Biol Reprod. 2021. PMID: 34518881 Free PMC article.
References
-
- Yanagimachi R. Mammalian fertilization. Knobil E, Neill J. (eds.), The Physiology of Reproduction. New York: Raven Press; 1994: 189 317
-
- LR Fraser. The “switching on” of mammalian spermatozoa: molecular events involved in promotion and regulation of capacitation. Mol Reprod Dev 2010; 77: 197 208 - PubMed
-
- Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol 2002; 53: 133 150 - PubMed
-
- Fraser L. Motility patterns in mouse spermatozoa before and after capacitation. J Exp Zool 1977; 202: 439 444 - PubMed
-
- Suarez SS, Osman RA. Initiation of hyperactivated flagellar bending in mouse sperm within the female reproductive tract. Biol Reprod 1987; 36: 1191 1198 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

