Use of an adult rat retinal explant model for screening of potential retinal ganglion cell neuroprotective therapies
- PMID: 21345987
- PMCID: PMC3109030
- DOI: 10.1167/iovs.10-6873
Use of an adult rat retinal explant model for screening of potential retinal ganglion cell neuroprotective therapies
Abstract
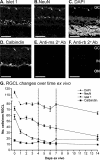
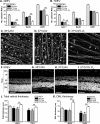
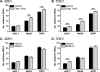
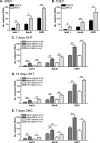
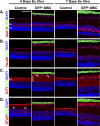
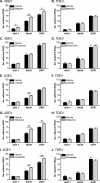
PURPOSE. To validate an established adult organotypic retinal explant culture system for use as an efficient medium-throughput screening tool to investigate novel retinal ganglion cell (RGC) neuroprotective therapies. METHODS. Optimal culture conditions for detecting RGC neuroprotection in rat retinal explants were identified. Retinal explants were treated with various recognized, or purported, neuroprotective agents and cultured for either 4 or 7 days ex vivo. The number of cells surviving in the RGC layer (RGCL) was quantified using histologic and immunohistochemical techniques, and statistical analyses were applied to detect neuroprotective effects. RESULTS. The ability to replicate previously reported in vivo RGC neuroprotection in retinal explants was verified by demonstrating that caspase inhibition, brain-derived neurotrophic factor treatment, and stem cell transplantation all reduced RGCL cell loss in this model. Further screening of potential neuroprotective pharmacologic agents demonstrated that betaxolol, losartan, tafluprost, and simvastatin all alleviated RGCL cell loss in retinal explants, supporting previous reports. However, treatment with brimonidine did not protect RGCL neurons from death in retinal explant cultures. Explants cultured for 4 days ex vivo proved most sensitive for detecting neuroprotection. CONCLUSIONS. The current adult rat retinal explant culture model offers advantages over other models for screening potential neuroprotective drugs, including maintenance of neurons in situ, control of environmental conditions, and dissociation from other factors such as intraocular pressure. Verification that neuroprotection by previously identified RGC-protective therapies could be replicated in adult retinal explant cultures suggests that this model could be used for efficient medium-throughput screening of novel neuroprotective therapies for retinal neurodegenerative disease.
Figures
Similar articles
-
Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome.Brain. 2014 Feb;137(Pt 2):503-19. doi: 10.1093/brain/awt292. Epub 2013 Oct 30. Brain. 2014. PMID: 24176979 Free PMC article.
-
A mouse retinal explant model for use in studying neuroprotection in glaucoma.Exp Eye Res. 2016 Oct;151:38-44. doi: 10.1016/j.exer.2016.07.010. Epub 2016 Jul 21. Exp Eye Res. 2016. PMID: 27450912
-
Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells.Invest Ophthalmol Vis Sci. 2010 Jan;51(1):526-34. doi: 10.1167/iovs.09-3903. Epub 2009 Jul 23. Invest Ophthalmol Vis Sci. 2010. PMID: 19628741
-
Diversified Treatment Options of Adult Stem Cells for Optic Neuropathies.Cell Transplant. 2022 Jan-Dec;31:9636897221123512. doi: 10.1177/09636897221123512. Cell Transplant. 2022. PMID: 36165292 Free PMC article. Review.
-
Transplantation prospects for the inner retina.Eye (Lond). 2009 Oct;23(10):1980-4. doi: 10.1038/eye.2008.376. Epub 2008 Dec 19. Eye (Lond). 2009. PMID: 19098702 Review.
Cited by
-
In Vitro and In Vivo Proteomic Comparison of Human Neural Progenitor Cell-Induced Photoreceptor Survival.Proteomics. 2019 Feb;19(3):e1800213. doi: 10.1002/pmic.201800213. Epub 2019 Jan 2. Proteomics. 2019. PMID: 30515959 Free PMC article.
-
NMOSD IgG Impact Retinal Cells in Murine Retinal Explants.Curr Issues Mol Biol. 2023 Sep 7;45(9):7319-7335. doi: 10.3390/cimb45090463. Curr Issues Mol Biol. 2023. PMID: 37754247 Free PMC article.
-
Viability of mitochondria-labeled retinal ganglion cells in organotypic retinal explant cultures by two methods.Exp Eye Res. 2023 Jan;226:109311. doi: 10.1016/j.exer.2022.109311. Epub 2022 Nov 17. Exp Eye Res. 2023. PMID: 36403849 Free PMC article.
-
Evaluation of neuroprotective and immunomodulatory properties of mesenchymal stem cells in an ex vivo retinal explant model.J Neuroinflammation. 2022 Mar 2;19(1):63. doi: 10.1186/s12974-022-02418-w. J Neuroinflammation. 2022. PMID: 35236378 Free PMC article.
-
A new and reliable guide for studies of neuronal loss based on focal lesions and combinations of in vivo and in vitro approaches.PLoS One. 2013 Apr 9;8(4):e60486. doi: 10.1371/journal.pone.0060486. Print 2013. PLoS One. 2013. PMID: 23585836 Free PMC article.
References
-
- Zhang SS, Fu XY, Barnstable CJ. Tissue culture studies of retinal development. Methods. 2002;28:439–447 - PubMed
-
- Bermel C, Tonges L, Planchamp V, et al. Combined inhibition of Cdk5 and ROCK additively increase cell survival, but not the regenerative response in regenerating retinal ganglion cells. Mol Cell Neurosci. 2009;42:427–437 - PubMed
-
- Feigenspan A, Dedek K, Schlich K, Weiler R, Thanos S. Expression and biophysical characterization of voltage-gated sodium channels in axons and growth cones of the regenerating optic nerve. Invest Ophthalmol Vis Sci. 2010;51:1789–1799 - PubMed
-
- Leaver SG, Harvey AR, Plant GW. Adult olfactory ensheathing glia promote the long-distance growth of adult retinal ganglion cell neurites in vitro. Glia. 2006;53:467–476 - PubMed
-
- Manabe S, Kashii S, Honda Y, Yamamoto R, Katsuki H, Akaike A. Quantification of axotomized ganglion cell death by explant culture of the rat retina. Neurosci Lett. 2002;334:33–36 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

