Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques
- PMID: 21339485
- PMCID: PMC3131163
- DOI: 10.1161/CIRCULATIONAHA.110.984146
Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques
Abstract
Background: We previously showed that the progression of atherosclerosis in the Reversa mouse (Ldlr(-/-Apob100/100Mttpfl/fl) Mx1Cre(+/+)) was arrested when the hyperlipidemia was normalized by inactivating the gene for microsomal triglyceride transfer protein. Here, we tested whether atherosclerosis would regress if the lipid levels were reduced after advanced plaques formed.
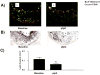
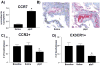
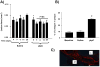
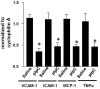
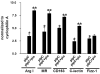
Methods and results: Reversa mice were fed an atherogenic diet for 16 weeks. Plasma lipid levels were then reduced. Within 2 weeks, this reduction led to decreased monocyte-derived (CD68(+)) cells in atherosclerotic plaques and was associated with emigration of these cells out of plaques. In addition, the fall in lipid levels was accompanied by lower plaque lipid content and by reduced expression in plaque CD68(+) cells of inflammatory genes and higher expression of genes for markers of antiinflammatory M2 macrophages. Plaque composition was affected more than plaque size, with the decreased content of lipid and CD68(+) cells balanced by a higher content of collagen. When the reduced lipid level was combined with the administration of pioglitazone to simulate the clinical aggressive lipid management and proliferator-activated receptor-γ agonist treatment, the rate of depletion of plaque CD68(+) cells was unaffected, but there was a further increase in their expression of antiinflammatory macrophage markers.
Conclusion: The Reversa mouse is a new model of atherosclerosis regression. After lipid lowering, favorable changes in plaque composition were independent of changes in size. In addition, plaque CD68(+) cells became less inflammatory, an effect enhanced by treatment with pioglitazone.
Figures
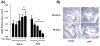

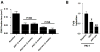
Similar articles
-
Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice.Diabetes. 2011 Jun;60(6):1759-69. doi: 10.2337/db10-0778. Epub 2011 May 11. Diabetes. 2011. PMID: 21562077 Free PMC article.
-
Effects of High Fat Feeding and Diabetes on Regression of Atherosclerosis Induced by Low-Density Lipoprotein Receptor Gene Therapy in LDL Receptor-Deficient Mice.PLoS One. 2015 Jun 5;10(6):e0128996. doi: 10.1371/journal.pone.0128996. eCollection 2015. PLoS One. 2015. PMID: 26046657 Free PMC article.
-
Silencing Myeloid Netrin-1 Induces Inflammation Resolution and Plaque Regression.Circ Res. 2021 Aug 20;129(5):530-546. doi: 10.1161/CIRCRESAHA.121.319313. Epub 2021 Jul 22. Circ Res. 2021. PMID: 34289717 Free PMC article.
-
Macrophages in atherosclerosis: a dynamic balance.Nat Rev Immunol. 2013 Oct;13(10):709-21. doi: 10.1038/nri3520. Epub 2013 Sep 2. Nat Rev Immunol. 2013. PMID: 23995626 Free PMC article. Review.
-
The phenomenon of atherosclerosis reversal and regression: Lessons from animal models.Exp Mol Pathol. 2017 Feb;102(1):138-145. doi: 10.1016/j.yexmp.2017.01.013. Epub 2017 Jan 17. Exp Mol Pathol. 2017. PMID: 28108216 Review.
Cited by
-
Vascular function during prolonged progression and regression of atherosclerosis in mice.Arterioscler Thromb Vasc Biol. 2013 Mar;33(3):459-65. doi: 10.1161/ATVBAHA.112.252700. Epub 2013 Jan 10. Arterioscler Thromb Vasc Biol. 2013. PMID: 23307875 Free PMC article.
-
Rapid regression of atherosclerosis with MTP inhibitor treatment.Atherosclerosis. 2013 Mar;227(1):125-9. doi: 10.1016/j.atherosclerosis.2012.12.026. Epub 2013 Jan 1. Atherosclerosis. 2013. PMID: 23332773 Free PMC article.
-
Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice.PLoS One. 2013;8(1):e54625. doi: 10.1371/journal.pone.0054625. Epub 2013 Jan 22. PLoS One. 2013. PMID: 23349943 Free PMC article.
-
Macrophage polarization in metabolic disorders: functions and regulation.Curr Opin Lipidol. 2011 Oct;22(5):365-72. doi: 10.1097/MOL.0b013e32834a77b4. Curr Opin Lipidol. 2011. PMID: 21825981 Free PMC article. Review.
-
Phenotypic modulation of macrophages in response to plaque lipids.Curr Opin Lipidol. 2011 Oct;22(5):335-42. doi: 10.1097/MOL.0b013e32834a97e4. Curr Opin Lipidol. 2011. PMID: 21841486 Free PMC article. Review.
References
-
- Reis ED, Li J, Fayad ZA, Rong JX, Hansoty D, Aguinaldo JG, Fallon JT, Fisher EA. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein e-deficient mouse in a novel transplantation model. J Vasc Surg. 2001;34:541–547. - PubMed
-
- Trogan E, Fayad ZA, Itskovich VV, Aguinaldo JG, Mani V, Fallon JT, Chereshnev I, Fisher EA. Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arterioscler Thromb Vasc Biol. 2004;24:1714–1719. - PubMed
-
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. Ccr7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL087228/HL/NHLBI NIH HHS/United States
- F30 AG029748-04/AG/NIA NIH HHS/United States
- F30 AG029748/AG/NIA NIH HHS/United States
- F32HL087627/HL/NHLBI NIH HHS/United States
- R01 HL087228-05/HL/NHLBI NIH HHS/United States
- AG-029748/AG/NIA NIH HHS/United States
- R01 HL084312-07/HL/NHLBI NIH HHS/United States
- F32 HL087627/HL/NHLBI NIH HHS/United States
- P01 HL090553-04/HL/NHLBI NIH HHS/United States
- HL-084312/HL/NHLBI NIH HHS/United States
- R01 HL087228-01/HL/NHLBI NIH HHS/United States
- F32 HL087627-03/HL/NHLBI NIH HHS/United States
- T32HL098129/HL/NHLBI NIH HHS/United States
- P01 HL090553/HL/NHLBI NIH HHS/United States
- T32 HL098129/HL/NHLBI NIH HHS/United States
- T32 HL098129-03/HL/NHLBI NIH HHS/United States
- R01 HL084312/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

