Dynamic control of Cajal body number during zebrafish embryogenesis
- PMID: 21327108
- PMCID: PMC3035118
- DOI: 10.4161/nucl.1.1.10680
Dynamic control of Cajal body number during zebrafish embryogenesis
Abstract
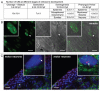
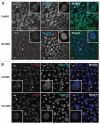
The Cajal body (CB) is an evolutionarily conserved nuclear subcompartment, enriched in components of the RNA processing machinery. The composition and dynamics of CBs in cells of living organisms is not well understood. Here we establish the zebrafish embryo as a model system to investigate the properties of CBs during rapid growth and cell division, taking advantage of the ease of live-cell imaging. We show that zebrafish embryo CBs contain coilin and multiple components of the pre-mRNA splicing machinery. Histone mRNA 3' end processing factors, present in CBs in some systems, were instead concentrated in a distinct nuclear body. CBs were present in embryos before and after activation of zygotic gene expression, indicating a maternal contribution of CB components. During the first 24 hours of development, embryonic cells displayed up to 30 CBs per nucleus; these dispersed prior to mitosis and reassembled within minutes upon daughter cell nucleus formation. Following zygotic genome activation, snRNP biogenesis was required for CB assembly and maintenance, suggesting a self-assembly process that determines CB numbers in embryos. Differentiation into muscle, neurons and epidermis was associated with the achievement of a steady state number of 2 CBs per nucleus. We propose that CB number is regulated during development to respond to the demands of gene expression in a rapidly growing embryo.
Keywords: Cajal body; pre-mRNA splicing; scaRNA; snRNA; snRNP; zygotic gene expression.
Figures


Similar articles
-
Activation of transcription enforces the formation of distinct nuclear bodies in zebrafish embryos.RNA Biol. 2017 Jun 3;14(6):752-760. doi: 10.1080/15476286.2016.1255397. Epub 2016 Nov 18. RNA Biol. 2017. PMID: 27858508 Free PMC article. Review.
-
Cajal bodies in neurons.RNA Biol. 2017 Jun 3;14(6):712-725. doi: 10.1080/15476286.2016.1231360. Epub 2016 Sep 14. RNA Biol. 2017. PMID: 27627892 Free PMC article. Review.
-
The Cajal body: a meeting place for spliceosomal snRNPs in the nuclear maze.Chromosoma. 2006 Oct;115(5):343-54. doi: 10.1007/s00412-006-0056-6. Epub 2006 Mar 31. Chromosoma. 2006. PMID: 16575476 Review.
-
The Cajal body and histone locus body.Cold Spring Harb Perspect Biol. 2010 Jul;2(7):a000653. doi: 10.1101/cshperspect.a000653. Epub 2010 May 26. Cold Spring Harb Perspect Biol. 2010. PMID: 20504965 Free PMC article. Review.
-
Assembly of snRNP-containing coiled bodies is regulated in interphase and mitosis--evidence that the coiled body is a kinetic nuclear structure.J Cell Biol. 1993 Feb;120(4):841-52. doi: 10.1083/jcb.120.4.841. J Cell Biol. 1993. PMID: 7679389 Free PMC article.
Cited by
-
Phosphorylation and the Cajal body: modification in search of function.Arch Biochem Biophys. 2010 Apr 15;496(2):69-76. doi: 10.1016/j.abb.2010.02.012. Epub 2010 Mar 1. Arch Biochem Biophys. 2010. PMID: 20193656 Free PMC article. Review.
-
Coilin, the signature protein of Cajal bodies, differentially modulates the interactions of plants with viruses in widely different taxa.Nucleus. 2014 Jan-Feb;5(1):85-94. doi: 10.4161/nucl.28315. Epub 2014 Feb 24. Nucleus. 2014. PMID: 24637832 Free PMC article.
-
Whole-genome screening identifies proteins localized to distinct nuclear bodies.J Cell Biol. 2013 Oct 14;203(1):149-64. doi: 10.1083/jcb.201303145. J Cell Biol. 2013. PMID: 24127217 Free PMC article.
-
Diabetic polyneuropathy, sensory neurons, nuclear structure and spliceosome alterations: a role for CWC22.Dis Model Mech. 2017 Mar 1;10(3):215-224. doi: 10.1242/dmm.028225. Dis Model Mech. 2017. PMID: 28250049 Free PMC article.
-
Coilin phosphomutants disrupt Cajal body formation, reduce cell proliferation and produce a distinct coilin degradation product.PLoS One. 2011;6(10):e25743. doi: 10.1371/journal.pone.0025743. Epub 2011 Oct 3. PLoS One. 2011. PMID: 21991343 Free PMC article.
References
-
- Gall JG. Cajal bodies: the first 100 years. Annu Rev Cell Dev Biol. 2000;16:14–16. - PubMed
-
- Raska I, Andrade LE, Ochs RL, Chan EK, Chang CM, Roos G, et al. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991;195:27–37. - PubMed
-
- Tucker KE, Massello LK, Gao L, Barber TJ, Hebert MD, Chan EK, et al. Structure and characterization of the murine p80 coilin gene, Coil. J Struct Biol. 2000;129:269–277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
