Rotigotine effects on early morning motor function and sleep in Parkinson's disease: a double-blind, randomized, placebo-controlled study (RECOVER)
- PMID: 21322021
- PMCID: PMC3072524
- DOI: 10.1002/mds.23441
Rotigotine effects on early morning motor function and sleep in Parkinson's disease: a double-blind, randomized, placebo-controlled study (RECOVER)
Abstract
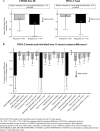
In a multinational, double-blind, placebo-controlled trial (NCT00474058), 287 subjects with Parkinson's disease (PD) and unsatisfactory early-morning motor symptom control were randomized 2:1 to receive rotigotine (2-16 mg/24 hr [n = 190]) or placebo (n = 97). Treatment was titrated to optimal dose over 1-8 weeks with subsequent dose maintenance for 4 weeks. Early-morning motor function and nocturnal sleep disturbance were assessed as coprimary efficacy endpoints using the Unified Parkinson's Disease Rating Scale (UPDRS) Part III (Motor Examination) measured in the early morning prior to any medication intake and the modified Parkinson's Disease Sleep Scale (PDSS-2) (mean change from baseline to end of maintenance [EOM], last observation carried forward). At EOM, mean UPDRS Part III score had decreased by -7.0 points with rotigotine (from a baseline of 29.6 [standard deviation (SD) 12.3] and by -3.9 points with placebo (baseline 32.0 [13.3]). Mean PDSS-2 total score had decreased by -5.9 points with rotigotine (from a baseline of 19.3 [SD 9.3]) and by -1.9 points with placebo (baseline 20.5 [10.4]). This represented a significantly greater improvement with rotigotine compared with placebo on both the UPDRS Part III (treatment difference: -3.55 [95% confidence interval (CI) -5.37, -1.73]; P = 0.0002) and PDSS-2 (treatment difference: -4.26 [95% CI -6.08, -2.45]; P < 0.0001). The most frequently reported adverse events were nausea (placebo, 9%; rotigotine, 21%), application site reactions (placebo, 4%; rotigotine, 15%), and dizziness (placebo, 6%; rotigotine 10%). Twenty-four-hour transdermal delivery of rotigotine to PD patients with early-morning motor dysfunction resulted in significant benefits in control of both motor function and nocturnal sleep disturbances.
Copyright © 2010 Movement Disorder Society.
Figures

Comment in
-
Parkinson disease: Skin patch improves sleep in PD.Nat Rev Neurol. 2011 Feb;7(2):64. doi: 10.1038/nrneurol.2010.208. Nat Rev Neurol. 2011. PMID: 21391321 No abstract available.
Similar articles
-
Associations between severity of motor function and nonmotor symptoms in Parkinson's disease: a post hoc analysis of the RECOVER Study.Eur Neurol. 2014;71(3-4):140-7. doi: 10.1159/000355019. Epub 2014 Jan 21. Eur Neurol. 2014. PMID: 24457253 Clinical Trial.
-
Rotigotine may improve sleep architecture in Parkinson's disease: a double-blind, randomized, placebo-controlled polysomnographic study.Sleep Med. 2016 May;21:140-4. doi: 10.1016/j.sleep.2016.01.016. Epub 2016 Feb 17. Sleep Med. 2016. PMID: 27448485 Clinical Trial.
-
Rotigotine transdermal system and evaluation of pain in patients with Parkinson's disease: a post hoc analysis of the RECOVER study.BMC Neurol. 2014 Mar 6;14:42. doi: 10.1186/1471-2377-14-42. BMC Neurol. 2014. PMID: 24602411 Free PMC article. Clinical Trial.
-
Rotigotine transdermal patch: a review of its use in the treatment of Parkinson's disease.CNS Drugs. 2011 Aug;25(8):699-719. doi: 10.2165/11206750-000000000-00000. CNS Drugs. 2011. PMID: 21790211 Review.
-
Rotigotine transdermal system for the treatment of Parkinson's disease.Clin Ther. 2008 May;30(5):813-24. doi: 10.1016/j.clinthera.2008.05.007. Clin Ther. 2008. PMID: 18555929 Review.
Cited by
-
The safety and tolerability of rotigotine transdermal system over a 6-year period in patients with early-stage Parkinson's disease.J Neural Transm (Vienna). 2013 Sep;120(9):1321-9. doi: 10.1007/s00702-013-1001-5. Epub 2013 Mar 19. J Neural Transm (Vienna). 2013. PMID: 23508526 Clinical Trial.
-
Management of Pain in Parkinson's Disease.J Parkinsons Dis. 2020;10(s1):S37-S48. doi: 10.3233/JPD-202069. J Parkinsons Dis. 2020. PMID: 32568113 Free PMC article. Review.
-
Pains in Parkinson disease--many syndromes under one umbrella.Nat Rev Neurol. 2012 Apr 17;8(5):284-94. doi: 10.1038/nrneurol.2012.54. Nat Rev Neurol. 2012. PMID: 22508236 Review.
-
Non-oral Continuous Drug Delivery Techniques in Parkinson's Disease: For Whom, When, and How?Mov Disord Clin Pract. 2016 Mar 24;3(3):221-229. doi: 10.1002/mdc3.12303. eCollection 2016 May-Jun. Mov Disord Clin Pract. 2016. PMID: 30363573 Free PMC article. Review.
-
Rotigotine Transdermal Patch: A Review in Parkinson's Disease.CNS Drugs. 2019 Jul;33(7):707-718. doi: 10.1007/s40263-019-00646-y. CNS Drugs. 2019. PMID: 31243728 Review.
References
-
- Poewe W. Non-motor symptoms in Parkinson's disease. Eur J Neurol. 2008;15(Suppl 1):14–20. - PubMed
-
- Wolters EC, Tesselaar HJ. International (NL-UK) double-blind study of Sinemet CR and standard Sinemet (25/100) in 170 patients with fluctuating Parkinson's disease. J Neurol. 1996;243:235–240. - PubMed
-
- Högl B, Rothdach A, Wetter TC, Trenkwalder C. The effect of cabergoline on sleep, periodic leg movements in sleep, and early morning motor function in patients with Parkinson's disease. Neuropsychopharmacology. 2003;28:1866–1870. - PubMed
-
- Romigi A, Stanzione P, Marciani MG, et al. Effect of cabergoline added to levodopa treatment on sleep-wake cycle in idiopathic Parkinson's disease: an open label 24-hour polysomnographic study. J Neural Transm. 2006;113:1909–1913. - PubMed
-
- Rektorova I, Balaz M, Svatova J, et al. Effects of ropinirole on nonmotor symptoms of Parkinson disease: a prospective multicenter study. Clin Neuropharmacol. 2008;31:261–266. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

