Chemical contrast for imaging living systems: molecular vibrations drive CARS microscopy
- PMID: 21321552
- PMCID: PMC7098185
- DOI: 10.1038/nchembio.525
Chemical contrast for imaging living systems: molecular vibrations drive CARS microscopy
Abstract
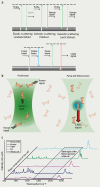
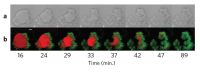
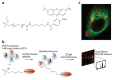
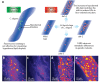
Cellular biomolecules contain unique molecular vibrations that can be visualized by coherent anti-Stokes Raman scattering (CARS) microscopy without the need for labels. Here we review the application of CARS microscopy for label-free imaging of cells and tissues using the natural vibrational contrast that arises from biomolecules like lipids as well as for imaging of exogenously added probes or drugs. High-resolution CARS microscopy combined with multimodal imaging has allowed for dynamic monitoring of cellular processes such as lipid metabolism and storage, the movement of organelles, adipogenesis and host-pathogen interactions and can also be used to track molecules within cells and tissues. The CARS imaging modality provides a unique tool for biological chemists to elucidate the state of a cellular environment without perturbing it and to perceive the functional effects of added molecules.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Label-free cellular imaging by broadband coherent anti-Stokes Raman scattering microscopy.Biophys J. 2010 Oct 20;99(8):2695-704. doi: 10.1016/j.bpj.2010.08.009. Biophys J. 2010. PMID: 20959111 Free PMC article.
-
Live cell imaging with chemical specificity using dual frequency CARS microscopy.Methods Enzymol. 2012;504:273-91. doi: 10.1016/B978-0-12-391857-4.00014-8. Methods Enzymol. 2012. PMID: 22264540 Review.
-
Fast vibrational imaging of single cells and tissues by stimulated Raman scattering microscopy.Acc Chem Res. 2014 Aug 19;47(8):2282-90. doi: 10.1021/ar400331q. Epub 2014 May 28. Acc Chem Res. 2014. PMID: 24871269 Free PMC article.
-
Label-free imaging of lipid dynamics using Coherent Anti-stokes Raman Scattering (CARS) and Stimulated Raman Scattering (SRS) microscopy.Curr Opin Genet Dev. 2011 Oct;21(5):585-90. doi: 10.1016/j.gde.2011.09.003. Epub 2011 Sep 22. Curr Opin Genet Dev. 2011. PMID: 21945002 Free PMC article. Review.
-
Coherent anti-stokes Raman scattering microscopy for high speed non- staining biomolecular imaging.Curr Pharm Biotechnol. 2013;14(2):150-8. Curr Pharm Biotechnol. 2013. PMID: 22356111 Review.
Cited by
-
Empagliflozin Preserves Skeletal Muscle Function in a HFpEF Rat Model.Int J Mol Sci. 2022 Sep 20;23(19):10989. doi: 10.3390/ijms231910989. Int J Mol Sci. 2022. PMID: 36232292 Free PMC article.
-
Structural, electronic and nonlinear optical properties, reactivity and solubility of the drug dihydroartemisinin functionalized on the carbon nanotube.Heliyon. 2023 Jan 2;9(1):e12663. doi: 10.1016/j.heliyon.2022.e12663. eCollection 2023 Jan. Heliyon. 2023. PMID: 36632106 Free PMC article.
-
Nonlinear Optical Methods for Characterization of Molecular Structure and Surface Chemistry.Top Catal. 2018 Jun;61(9-11):1101-1124. doi: 10.1007/s11244-018-0924-3. Epub 2018 Apr 17. Top Catal. 2018. PMID: 29955207 Free PMC article.
-
Inside single cells: quantitative analysis with advanced optics and nanomaterials.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015 May-Jun;7(3):387-407. doi: 10.1002/wnan.1321. Epub 2014 Nov 27. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015. PMID: 25430077 Free PMC article. Review.
-
Synchronization-free all-solid-state laser system for stimulated Raman scattering microscopy.Light Sci Appl. 2016 Oct 7;5(10):e16149. doi: 10.1038/lsa.2016.149. eCollection 2016 Oct. Light Sci Appl. 2016. PMID: 30167121 Free PMC article.
References
-
- Prescher JA, Bertozzi CR. Chemistry in living systems. Nat. Chem. Biol. 2005;1:13–21. - PubMed
-
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 2003;9:702–712. - PubMed
-
- Zaret KS. Regulatory phases of early liver development: Paradigms of organogenesis. Nat. Rev. Genet. 2002;3:499–512. - PubMed
-
- Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. The complete atomic structure of the large ribosomal subunit at 2.4 angstrom resolution. Science. 2000;289:905–920. - PubMed
-
- Fischer N, Konevega AL, Wintermeyer W, Rodnina MV, Stark H. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature. 2010;466:329–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

