Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells
- PMID: 21321227
- PMCID: PMC3048161
- DOI: 10.1073/pnas.1017275108
Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells
Abstract
Deposition of proteins of aberrant conformation is the hallmark of many neurodegenerative diseases. Misfolding of the normally globular mutant superoxide dismutase-1 (SOD1) is a central, early, but poorly understood event in the pathogenic cascade leading to familial forms of ALS. Here we report that aggregates composed of an ALS-causing SOD1 mutant penetrate inside cells by macropinocytosis and rapidly exit the macropinocytic compartment to nucleate aggregation of the cytosolic, otherwise soluble, mutant SOD1 protein. Once initiated, mutant SOD1 aggregation is self-perpetuating. Mutant SOD1 aggregates transfer from cell to cell with remarkable efficiency, a process that does not require contacts between cells but depends on the extracellular release of aggregates. This study reveals that SOD1 aggregates, propagate in a prion-like manner in neuronal cells and sheds light on the mechanisms underlying aggregate uptake and cell-to-cell transfer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
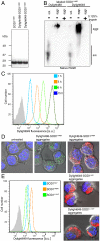
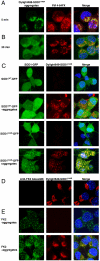
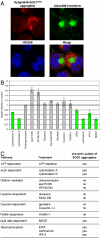
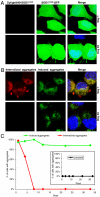
Comment in
-
Self-propagation and transmission of misfolded mutant SOD1: prion or prion-like phenomenon?Cell Cycle. 2011 Jun 1;10(11):1711. doi: 10.4161/cc.10.11.15560. Epub 2011 Jun 1. Cell Cycle. 2011. PMID: 21471733 No abstract available.
Similar articles
-
Exosome-dependent and independent mechanisms are involved in prion-like transmission of propagated Cu/Zn superoxide dismutase misfolding.Prion. 2014;8(5):331-5. doi: 10.4161/19336896.2014.983398. Prion. 2014. PMID: 25551548 Free PMC article.
-
Superoxide dismutase 1 encoding mutations linked to ALS adopts a spectrum of misfolded states.Mol Neurodegener. 2011 Nov 17;6:77. doi: 10.1186/1750-1326-6-77. Mol Neurodegener. 2011. PMID: 22094223 Free PMC article.
-
Prion-like activity of Cu/Zn superoxide dismutase: implications for amyotrophic lateral sclerosis.Prion. 2014 Jan-Feb;8(1):33-41. doi: 10.4161/pri.27602. Prion. 2014. PMID: 24394345 Free PMC article.
-
The prion-like nature of amyotrophic lateral sclerosis.Prog Mol Biol Transl Sci. 2020;175:261-296. doi: 10.1016/bs.pmbts.2020.07.002. Epub 2020 Sep 1. Prog Mol Biol Transl Sci. 2020. PMID: 32958236 Review.
-
From molecule to molecule and cell to cell: prion-like mechanisms in amyotrophic lateral sclerosis.Neurobiol Dis. 2015 May;77:257-65. doi: 10.1016/j.nbd.2015.02.009. Epub 2015 Feb 17. Neurobiol Dis. 2015. PMID: 25701498 Review.
Cited by
-
Proteostatic imbalance and protein spreading in amyotrophic lateral sclerosis.EMBO J. 2021 May 17;40(10):e106389. doi: 10.15252/embj.2020106389. Epub 2021 Mar 31. EMBO J. 2021. PMID: 33792056 Free PMC article. Review.
-
Thinking laterally about neurodegenerative proteinopathies.J Clin Invest. 2013 May;123(5):1847-55. doi: 10.1172/JCI66029. Epub 2013 May 1. J Clin Invest. 2013. PMID: 23635781 Free PMC article.
-
Exosome-dependent and independent mechanisms are involved in prion-like transmission of propagated Cu/Zn superoxide dismutase misfolding.Prion. 2014;8(5):331-5. doi: 10.4161/19336896.2014.983398. Prion. 2014. PMID: 25551548 Free PMC article.
-
Aggregate-selective antibody attenuates seeded aggregation but not spontaneously evolving disease in SOD1 ALS model mice.Acta Neuropathol Commun. 2020 Sep 14;8(1):161. doi: 10.1186/s40478-020-01032-2. Acta Neuropathol Commun. 2020. PMID: 32928301 Free PMC article.
-
Defining novel functions for cerebrospinal fluid in ALS pathophysiology.Acta Neuropathol Commun. 2020 Aug 20;8(1):140. doi: 10.1186/s40478-020-01018-0. Acta Neuropathol Commun. 2020. PMID: 32819425 Free PMC article. Review.
References
-
- Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Meyer-Luehmann M, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. - PubMed
-
- Yang W, Dunlap JR, Andrews RB, Wetzel R. Aggregated polyglutamine peptides delivered to nuclei are toxic to mammalian cells. Hum Mol Genet. 2002;11:2905–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

