Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine
- PMID: 21321204
- PMCID: PMC3048122
- DOI: 10.1073/pnas.1014033108
Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine
Abstract
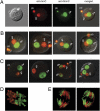
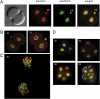
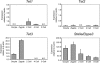
Genome-wide erasure of DNA cytosine-5 methylation has been reported to occur along the paternal pronucleus in fertilized oocytes in an apparently replication-independent manner, but the mechanism of this reprogramming process has remained enigmatic. Recently, considerable amounts of 5-hydroxymethylcytosine (5hmC), most likely derived from enzymatic oxidation of 5-methylcytosine (5mC) by TET proteins, have been detected in certain mammalian tissues. 5hmC has been proposed as a potential intermediate in active DNA demethylation. Here, we show that in advanced pronuclear-stage zygotes the paternal pronucleus contains substantial amounts of 5hmC but lacks 5mC. The converse is true for the maternal pronucleus, which retains 5mC but shows little or no 5hmC signal. Importantly, 5hmC persists into mitotic one-cell, two-cell, and later cleavage-stage embryos, suggesting that 5mC oxidation is not followed immediately by genome-wide removal of 5hmC through excision repair pathways or other mechanisms. This conclusion is supported by bisulfite sequencing data, which shows only limited conversion of modified cytosines to cytosines at several gene loci. It is likely that 5mC oxidation is carried out by the Tet3 oxidase. Tet3, but not Tet1 or Tet2, was expressed at high levels in oocytes and zygotes, with rapidly declining levels at the two-cell stage. Our results show that 5mC oxidation is part of the early life cycle of mammals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming.Nat Commun. 2011;2:241. doi: 10.1038/ncomms1240. Nat Commun. 2011. PMID: 21407207
-
The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes.Nature. 2011 Sep 4;477(7366):606-10. doi: 10.1038/nature10443. Nature. 2011. PMID: 21892189
-
Comparative dynamics of 5-methylcytosine reprogramming and TET family expression during preimplantation mammalian development in mouse and sheep.Theriogenology. 2017 Feb;89:86-96. doi: 10.1016/j.theriogenology.2016.10.010. Epub 2016 Oct 20. Theriogenology. 2017. PMID: 28043375
-
5-Hydroxymethylcytosine: a stable or transient DNA modification?Genomics. 2014 Nov;104(5):314-23. doi: 10.1016/j.ygeno.2014.08.015. Epub 2014 Aug 30. Genomics. 2014. PMID: 25181633 Free PMC article. Review.
-
Genomic distribution and possible functions of DNA hydroxymethylation in the brain.Genomics. 2014 Nov;104(5):341-6. doi: 10.1016/j.ygeno.2014.08.020. Epub 2014 Sep 7. Genomics. 2014. PMID: 25205307 Review.
Cited by
-
Tet family of 5-methylcytosine dioxygenases in mammalian development.J Hum Genet. 2013 Jul;58(7):421-7. doi: 10.1038/jhg.2013.63. Epub 2013 May 30. J Hum Genet. 2013. PMID: 23719188 Free PMC article. Review.
-
Stage-specific roles for tet1 and tet2 in DNA demethylation in primordial germ cells.Cell Stem Cell. 2013 Apr 4;12(4):470-8. doi: 10.1016/j.stem.2013.01.016. Epub 2013 Feb 14. Cell Stem Cell. 2013. PMID: 23415914 Free PMC article.
-
Genotype-independent transmission of transgenic fluorophore protein by boar spermatozoa.PLoS One. 2011;6(11):e27563. doi: 10.1371/journal.pone.0027563. Epub 2011 Nov 16. PLoS One. 2011. PMID: 22110672 Free PMC article.
-
5-hydroxymethylcytosine and its potential roles in development and cancer.Epigenetics Chromatin. 2013 May 1;6(1):10. doi: 10.1186/1756-8935-6-10. Epigenetics Chromatin. 2013. PMID: 23634848 Free PMC article.
-
PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos.Nature. 2012 Jun 3;486(7403):415-9. doi: 10.1038/nature11093. Nature. 2012. PMID: 22722204
References
-
- Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–232. - PubMed
-
- Riggs AD. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14:9–25. - PubMed
-
- Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441:1061–1067. - PubMed
-
- Huang K, Fan G. DNA methylation in cell differentiation and reprogramming: An emerging systematic view. Regen Med. 2010;5:531–544. - PubMed
-
- Straussman R, et al. Developmental programming of CpG island methylation profiles in the human genome. Nat Struct Mol Biol. 2009;16:564–571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

