Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens
- PMID: 21307853
- PMCID: PMC3076739
- DOI: 10.1038/nature09849
Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens
Abstract
Under physiological conditions the gut-associated lymphoid tissues not only prevent the induction of a local inflammatory immune response, but also induce systemic tolerance to fed antigens. A notable exception is coeliac disease, where genetically susceptible individuals expressing human leukocyte antigen (HLA) HLA-DQ2 or HLA-DQ8 molecules develop inflammatory T-cell and antibody responses against dietary gluten, a protein present in wheat. The mechanisms underlying this dysregulated mucosal immune response to a soluble antigen have not been identified. Retinoic acid, a metabolite of vitamin A, has been shown to have a critical role in the induction of intestinal regulatory responses. Here we find in mice that in conjunction with IL-15, a cytokine greatly upregulated in the gut of coeliac disease patients, retinoic acid rapidly activates dendritic cells to induce JNK (also known as MAPK8) phosphorylation and release the proinflammatory cytokines IL-12p70 and IL-23. As a result, in a stressed intestinal environment, retinoic acid acted as an adjuvant that promoted rather than prevented inflammatory cellular and humoral responses to fed antigen. Altogether, these findings reveal an unexpected role for retinoic acid and IL-15 in the abrogation of tolerance to dietary antigens.
Figures
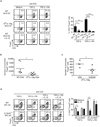
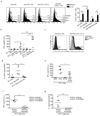
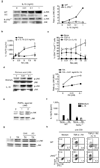

Comment in
-
Immunology: Context is key in the gut.Nature. 2011 Mar 10;471(7337):169-70. doi: 10.1038/471169a. Nature. 2011. PMID: 21390118 No abstract available.
-
Mucosal immunology: Acid attack.Nat Rev Immunol. 2011 Mar;11(3):156. doi: 10.1038/nri2948. Nat Rev Immunol. 2011. PMID: 21452586 No abstract available.
-
Celiac disease: Retinoic acid and IL-15 jointly implicated in reversal of oral tolerance.Nat Rev Gastroenterol Hepatol. 2011 Apr;8(4):181. doi: 10.1038/nrgastro.2011.29. Nat Rev Gastroenterol Hepatol. 2011. PMID: 21595057 No abstract available.
-
IL-15 modulates the effect of retinoic acid, promoting inflammation rather than oral tolerance to dietary antigens.Expert Rev Gastroenterol Hepatol. 2011 Jun;5(3):315-7. doi: 10.1586/egh.11.36. Expert Rev Gastroenterol Hepatol. 2011. PMID: 21651349
Similar articles
-
Immunology: Context is key in the gut.Nature. 2011 Mar 10;471(7337):169-70. doi: 10.1038/471169a. Nature. 2011. PMID: 21390118 No abstract available.
-
Gliadin Nanoparticles Induce Immune Tolerance to Gliadin in Mouse Models of Celiac Disease.Gastroenterology. 2020 May;158(6):1667-1681.e12. doi: 10.1053/j.gastro.2020.01.045. Epub 2020 Feb 4. Gastroenterology. 2020. PMID: 32032584 Free PMC article.
-
IL-15, gluten and HLA-DQ8 drive tissue destruction in coeliac disease.Nature. 2020 Feb;578(7796):600-604. doi: 10.1038/s41586-020-2003-8. Epub 2020 Feb 12. Nature. 2020. PMID: 32051586 Free PMC article.
-
Pathomechanisms in celiac disease.Int Arch Allergy Immunol. 2003 Oct;132(2):98-108. doi: 10.1159/000073710. Int Arch Allergy Immunol. 2003. PMID: 14600421 Review.
-
Celiac disease: how complicated can it get?Immunogenetics. 2010 Oct;62(10):641-51. doi: 10.1007/s00251-010-0465-9. Epub 2010 Jul 27. Immunogenetics. 2010. PMID: 20661732 Free PMC article. Review.
Cited by
-
Host and microbial factors in regulation of T cells in the intestine.Front Immunol. 2013 Jun 10;4:141. doi: 10.3389/fimmu.2013.00141. eCollection 2013. Front Immunol. 2013. PMID: 23772228 Free PMC article.
-
Retinoid and TGF-β families: crosstalk in development, neoplasia, immunity, and tissue repair.Semin Nephrol. 2012 May;32(3):287-94. doi: 10.1016/j.semnephrol.2012.04.008. Semin Nephrol. 2012. PMID: 22835460 Free PMC article. Review.
-
Sera of Neuromyelitis Optica Patients Increase BID-Mediated Apoptosis in Astrocytes.Int J Mol Sci. 2022 Jun 27;23(13):7117. doi: 10.3390/ijms23137117. Int J Mol Sci. 2022. PMID: 35806122 Free PMC article.
-
The light and dark sides of intestinal intraepithelial lymphocytes.Nat Rev Immunol. 2011 Jun 17;11(7):445-56. doi: 10.1038/nri3007. Nat Rev Immunol. 2011. PMID: 21681197 Free PMC article. Review.
-
Tissue adaptation: Implications for gut immunity and tolerance.J Exp Med. 2017 May 1;214(5):1211-1226. doi: 10.1084/jem.20162014. Epub 2017 Apr 21. J Exp Med. 2017. PMID: 28432200 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

