Endogenous retrovirus drives hitherto unknown proapoptotic p63 isoforms in the male germ line of humans and great apes
- PMID: 21300884
- PMCID: PMC3048127
- DOI: 10.1073/pnas.1016201108
Endogenous retrovirus drives hitherto unknown proapoptotic p63 isoforms in the male germ line of humans and great apes
Abstract
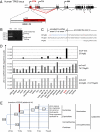
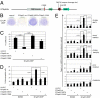
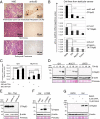
TAp63, but not its homolog p53, eliminates oocytes that suffered DNA damage. An equivalent gene for guarding the male germ line is currently not known. Here we identify hitherto unknown human p63 transcripts with unique 5'-ends derived from incorporated exons upstream of the currently mapped TP63 gene. These unique p63 transcripts are highly and specifically expressed in testis. Their most upstream region corresponds to a LTR of the human endogenous retrovirus 9 (ERV9). The insertion of this LTR upstream of the TP63 locus occurred only recently in evolution and is unique to humans and great apes (Hominidae). A corresponding p63 protein is the sole p63 species in healthy human testis, and is strongly expressed in spermatogenic precursors but not in mature spermatozoa. In response to DNA damage, this human male germ-cell-encoded TAp63 protein (designated GTAp63) is activated by caspase cleavage near its carboxyterminal domain and induces apoptosis. Human testicular cancer tissues and cell lines largely lost p63 expression. However, pharmacological inhibition of histone deacetylases completely restores p63 expression in testicular cancer cells (>3,000-fold increase). Our data support a model whereby testis-specific GTAp63 protects the genomic integrity of the male germ line and acts as a tumor suppressor. In Hominidae, this guardian function was greatly enhanced by integration of an endogenous retrovirus upstream of the TP63 locus that occurred 15 million years ago. By providing increased germ-line stability, this event may have contributed to the evolution of hominids and enabled their long reproductive periods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Comprehensive identification of genes driven by ERV9-LTRs reveals TNFRSF10B as a re-activatable mediator of testicular cancer cell death.Cell Death Differ. 2016 Jan;23(1):64-75. doi: 10.1038/cdd.2015.68. Epub 2015 May 29. Cell Death Differ. 2016. PMID: 26024393 Free PMC article.
-
Non-hominid TP63 lacks retroviral LTRs but contains a novel conserved upstream exon.Cell Cycle. 2011 Jun 15;10(12):1905-11. doi: 10.4161/cc.10.12.15838. Epub 2011 Jun 15. Cell Cycle. 2011. PMID: 21558811
-
Utility of next-generation RNA-sequencing in identifying chimeric transcription involving human endogenous retroviruses.APMIS. 2016 Jan-Feb;124(1-2):127-39. doi: 10.1111/apm.12477. APMIS. 2016. PMID: 26818267 Review.
-
Role of human endogenous retroviral long terminal repeats (LTRs) in maintaining the integrity of the human germ line.Viruses. 2011 Jun;3(6):901-5. doi: 10.3390/v3060901. Epub 2011 Jun 21. Viruses. 2011. PMID: 21994760 Free PMC article.
-
p63 the guardian of human reproduction.Cell Cycle. 2012 Dec 15;11(24):4545-51. doi: 10.4161/cc.22819. Epub 2012 Nov 19. Cell Cycle. 2012. PMID: 23165243 Free PMC article. Review.
Cited by
-
Efficacy of HDAC Inhibitors Belinostat and Panobinostat against Cisplatin-Sensitive and Cisplatin-Resistant Testicular Germ Cell Tumors.Cancers (Basel). 2020 Oct 10;12(10):2903. doi: 10.3390/cancers12102903. Cancers (Basel). 2020. PMID: 33050470 Free PMC article.
-
A novel function of RNAs arising from the long terminal repeat of human endogenous retrovirus 9 in cell cycle arrest.J Virol. 2013 Jan;87(1):25-36. doi: 10.1128/JVI.01648-12. Epub 2012 Oct 24. J Virol. 2013. PMID: 23097441 Free PMC article.
-
Epigenetic regulation of HERVs: Implications for cancer immunotherapy.Genes Genomics. 2024 Nov;46(11):1303-1312. doi: 10.1007/s13258-024-01546-2. Epub 2024 Aug 1. Genes Genomics. 2024. PMID: 39088189 Review.
-
Chk2 and p53 regulate the transmission of healed chromosomes in the Drosophila male germline.PLoS Genet. 2014 Feb 27;10(2):e1004130. doi: 10.1371/journal.pgen.1004130. eCollection 2014 Feb. PLoS Genet. 2014. PMID: 24586185 Free PMC article.
-
Endogenous Retroviruses: With Us and against Us.Front Chem. 2017 Apr 7;5:23. doi: 10.3389/fchem.2017.00023. eCollection 2017. Front Chem. 2017. PMID: 28439515 Free PMC article. Review.
References
-
- Yang A, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. - PubMed
-
- Yang A, McKeon F. P63 and P73: P53 mimics, menaces and more. Nat Rev Mol Cell Biol. 2000;1:199–207. - PubMed
-
- Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. - PubMed
-
- Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

