Contraction-induced skeletal muscle FAT/CD36 trafficking and FA uptake is AMPK independent
- PMID: 21297178
- PMCID: PMC3053206
- DOI: 10.1194/jlr.M007138
Contraction-induced skeletal muscle FAT/CD36 trafficking and FA uptake is AMPK independent
Abstract
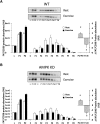
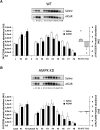
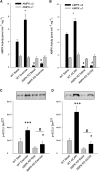
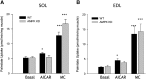
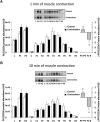
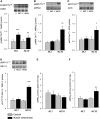
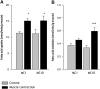
The aim of this study was to investigate the molecular mechanisms regulating FA translocase CD36 (FAT/CD36) translocation and FA uptake in skeletal muscle during contractions. In one model, wild-type (WT) and AMP-dependent protein kinase kinase dead (AMPK KD) mice were exercised or extensor digitorum longus (EDL) and soleus (SOL) muscles were contracted, ex vivo. In separate studies, FAT/CD36 translocation and FA uptake in response to muscle contractions were investigated in the perfused rat hindlimb. Exercise induced a similar increase in skeletal muscle cell surface membrane FAT/CD36 content in WT (+34%) and AMPK KD (+37%) mice. In contrast, 5-aminoimidazole-4-carboxamide ribonucleoside only induced an increase in cell surface FAT/CD36 content in WT (+29%) mice. Furthermore, in the perfused rat hindlimb, muscle contraction induced a rapid (1 min, +15%) and sustained (10 min, +24%) FAT/CD36 relocation to cell surface membranes. The increase in cell surface FAT/CD36 protein content with muscle contractions was associated with increased FA uptake, both in EDL and SOL muscle from WT and AMPK KD mice and in the perfused rat hindlimb. This suggests that AMPK is not essential in regulation of FAT/CD36 translocation and FA uptake in skeletal muscle during contractions. However, AMPK could be important in regulation of FAT/CD36 distribution in other physiological situations.
Figures

Similar articles
-
Contractions but not AICAR increase FABPpm content in rat muscle sarcolemma.Mol Cell Biochem. 2009 Jun;326(1-2):45-53. doi: 10.1007/s11010-008-0006-0. Epub 2009 Jan 14. Mol Cell Biochem. 2009. PMID: 19142713
-
A new leptin-mediated mechanism for stimulating fatty acid oxidation: a pivotal role for sarcolemmal FAT/CD36.Biochem J. 2017 Jan 1;474(1):149-162. doi: 10.1042/BCJ20160804. Epub 2016 Nov 8. Biochem J. 2017. PMID: 27827305
-
The alpha-subunit of AMPK is essential for submaximal contraction-mediated glucose transport in skeletal muscle in vitro.Am J Physiol Endocrinol Metab. 2008 Dec;295(6):E1447-54. doi: 10.1152/ajpendo.90362.2008. Epub 2008 Sep 23. Am J Physiol Endocrinol Metab. 2008. PMID: 18812461
-
Regulation of fatty acid transport by fatty acid translocase/CD36.Proc Nutr Soc. 2004 May;63(2):245-9. doi: 10.1079/PNS2004331. Proc Nutr Soc. 2004. PMID: 15294038 Review.
-
AMP-activated protein kinase control of fat metabolism in skeletal muscle.Acta Physiol (Oxf). 2009 May;196(1):147-54. doi: 10.1111/j.1748-1716.2009.01973.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245653 Free PMC article. Review.
Cited by
-
Exercise- and training-induced upregulation of skeletal muscle fatty acid oxidation are not solely dependent on mitochondrial machinery and biogenesis.J Physiol. 2013 Sep 15;591(18):4415-26. doi: 10.1113/jphysiol.2012.238451. Epub 2012 Aug 13. J Physiol. 2013. PMID: 22890711 Free PMC article. Review.
-
Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity.Proc Natl Acad Sci U S A. 2015 May 26;112(21):6748-53. doi: 10.1073/pnas.1504239112. Epub 2015 May 11. Proc Natl Acad Sci U S A. 2015. PMID: 25964318 Free PMC article.
-
AMPK and the Adaptation to Exercise.Annu Rev Physiol. 2022 Feb 10;84:209-227. doi: 10.1146/annurev-physiol-060721-095517. Annu Rev Physiol. 2022. PMID: 35143330 Free PMC article. Review.
-
CD36 protein influences myocardial Ca2+ homeostasis and phospholipid metabolism: conduction anomalies in CD36-deficient mice during fasting.J Biol Chem. 2012 Nov 9;287(46):38901-12. doi: 10.1074/jbc.M112.413609. Epub 2012 Sep 27. J Biol Chem. 2012. PMID: 23019328 Free PMC article.
-
Insulin and AMPK regulate FA translocase/CD36 plasma membrane recruitment in cardiomyocytes via Rab GAP AS160 and Rab8a Rab GTPase.J Lipid Res. 2012 Apr;53(4):709-17. doi: 10.1194/jlr.M023424. Epub 2012 Feb 6. J Lipid Res. 2012. PMID: 22315395 Free PMC article.
References
-
- Roepstorff C., Steffensen C. H., Madsen M., Stallknecht B., Kanstrup I. L., Richter E. A., Kiens B. 2002. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am. J. Physiol. Endocrinol. Metab. 282: E435–E447. - PubMed
-
- Stellingwerff T., Boon H., Jonkers R. A., Senden J. M., Spriet L. L., Koopman R., van Loon L. J. 2007. Significant intramyocellular lipid use during prolonged cycling in endurance-trained males as assessed by three different methodologies. Am. J. Physiol. Endocrinol. Metab. 292: E1715–E1723. - PubMed
-
- Romijn J. A., Coyle E. F., Sidossis L. S., Gastaldelli A., Horowitz J. F., Endert E., Wolfe R. R. 1993. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 265: E380–E391. - PubMed
-
- Burguera B., Proctor D., Dietz N., Guo Z., Joyner M., Jensen M. D. 2000. Leg free fatty acid kinetics during exercise in men and women. Am. J. Physiol. Endocrinol. Metab. 278: E113–E117. - PubMed
-
- Kiens B. 2006. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol. Rev. 86: 205–243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

