Linear ubiquitin assembly complex negatively regulates RIG-I- and TRIM25-mediated type I interferon induction
- PMID: 21292167
- PMCID: PMC3070481
- DOI: 10.1016/j.molcel.2010.12.029
Linear ubiquitin assembly complex negatively regulates RIG-I- and TRIM25-mediated type I interferon induction
Abstract
Upon detection of viral RNA, retinoic acid-inducible gene I (RIG-I) undergoes TRIM25-mediated K63-linked ubiquitination, leading to type I interferon (IFN) production. In this study, we demonstrate that the linear ubiquitin assembly complex (LUBAC), comprised of two RING-IBR-RING (RBR)-containing E3 ligases, HOIL-1L and HOIP, independently targets TRIM25 and RIG-I to effectively suppress virus-induced IFN production. RBR E3 ligase domains of HOIL-1L and HOIP bind and induce proteasomal degradation of TRIM25, whereas the NZF domain of HOIL-1L competes with TRIM25 for RIG-I binding. Consequently, both actions by the HOIL-1L/HOIP LUBAC potently inhibit RIG-I ubiquitination and antiviral activity, but in a mechanistically separate manner. Conversely, the genetic deletion or depletion of HOIL-1L and HOIP robustly enhances virus-induced type I IFN production. Taken together, the HOIL-1L/HOIP LUBAC specifically suppresses RIG-I ubiquitination and activation by inducing TRIM25 degradation and inhibiting TRIM25 interaction with RIG-I, resulting in the comprehensive suppression of the IFN-mediated antiviral signaling pathway.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

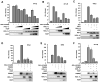

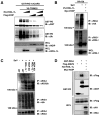


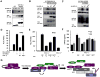
Similar articles
-
The ubiquitin-specific protease USP15 promotes RIG-I-mediated antiviral signaling by deubiquitylating TRIM25.Sci Signal. 2014 Jan 7;7(307):ra3. doi: 10.1126/scisignal.2004577. Sci Signal. 2014. PMID: 24399297 Free PMC article.
-
Activation of duck RIG-I by TRIM25 is independent of anchored ubiquitin.PLoS One. 2014 Jan 23;9(1):e86968. doi: 10.1371/journal.pone.0086968. eCollection 2014. PLoS One. 2014. PMID: 24466302 Free PMC article.
-
Mechanistic insights into the subversion of the linear ubiquitin chain assembly complex by the E3 ligase IpaH1.4 of Shigella flexneri.Proc Natl Acad Sci U S A. 2022 Mar 22;119(12):e2116776119. doi: 10.1073/pnas.2116776119. Epub 2022 Mar 16. Proc Natl Acad Sci U S A. 2022. PMID: 35294289 Free PMC article.
-
Ubiquitin-mediated modulation of the cytoplasmic viral RNA sensor RIG-I.J Biochem. 2012 Jan;151(1):5-11. doi: 10.1093/jb/mvr111. Epub 2011 Sep 2. J Biochem. 2012. PMID: 21890623 Review.
-
Linear Ubiquitin Code: Its Writer, Erasers, Decoders, Inhibitors, and Implications in Disorders.Int J Mol Sci. 2020 May 11;21(9):3381. doi: 10.3390/ijms21093381. Int J Mol Sci. 2020. PMID: 32403254 Free PMC article. Review.
Cited by
-
Hepatitis B Virus-Induced Parkin-Dependent Recruitment of Linear Ubiquitin Assembly Complex (LUBAC) to Mitochondria and Attenuation of Innate Immunity.PLoS Pathog. 2016 Jun 27;12(6):e1005693. doi: 10.1371/journal.ppat.1005693. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27348524 Free PMC article.
-
Multilayered regulations of RIG-I in the anti-viral signaling pathway.J Microbiol. 2016 Sep;54(9):583-587. doi: 10.1007/s12275-016-6322-2. Epub 2016 Aug 31. J Microbiol. 2016. PMID: 27572506 Review.
-
HOIL1 Is Essential for the Induction of Type I and III Interferons by MDA5 and Regulates Persistent Murine Norovirus Infection.J Virol. 2018 Nov 12;92(23):e01368-18. doi: 10.1128/JVI.01368-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30209176 Free PMC article.
-
Molecular control of the NEMO family of ubiquitin-binding proteins.Nat Rev Mol Cell Biol. 2013 Oct;14(10):673-85. doi: 10.1038/nrm3644. Epub 2013 Aug 29. Nat Rev Mol Cell Biol. 2013. PMID: 23989959 Review.
-
Trim25 Is an RNA-Specific Activator of Lin28a/TuT4-Mediated Uridylation.Cell Rep. 2014 Nov 20;9(4):1265-72. doi: 10.1016/j.celrep.2014.10.017. Cell Rep. 2014. PMID: 25457611 Free PMC article.
References
-
- Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI083025-02S1/AI/NIAID NIH HHS/United States
- R37 AI087846/AI/NIAID NIH HHS/United States
- AI083355/AI/NIAID NIH HHS/United States
- RR00168/RR/NCRR NIH HHS/United States
- AI083025/AI/NIAID NIH HHS/United States
- R01 CA082057/CA/NCI NIH HHS/United States
- U19 AI083025/AI/NIAID NIH HHS/United States
- CA082057/CA/NCI NIH HHS/United States
- U19 AI083025-03/AI/NIAID NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- K26 RR000168/RR/NCRR NIH HHS/United States
- R01 AI073099-05/AI/NIAID NIH HHS/United States
- AI087846-01A1/AI/NIAID NIH HHS/United States
- R01 AI087846/AI/NIAID NIH HHS/United States
- R01 AI073099/AI/NIAID NIH HHS/United States
- R01 AI083355-03/AI/NIAID NIH HHS/United States
- R01 AI083355/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

