Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis
- PMID: 21289221
- PMCID: PMC3057551
- DOI: 10.3945/ajcn.110.001917
Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis
Abstract
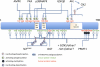
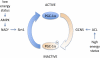
Mechanisms responsible for energy management in the cell and in the whole organism require a complex network of transcription factors and cofactors. Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) has emerged as a master regulator of mitochondrial biogenesis and function, thus becoming a crucial metabolic node. We present an overview of the mechanisms by which PGC-1α is regulated, including the transcriptional regulation of PGC-1α expression and the fine-tuning of its final activity via posttranslational modifications.
Figures

Similar articles
-
Mitochondrial biogenesis and peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) deacetylation by physical activity: intact adipocytokine signaling is required.Diabetes. 2011 Jan;60(1):157-67. doi: 10.2337/db10-0331. Epub 2010 Oct 7. Diabetes. 2011. PMID: 20929977 Free PMC article.
-
The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway.Adipocyte. 2020 Dec;9(1):484-494. doi: 10.1080/21623945.2020.1807850. Adipocyte. 2020. PMID: 32835596 Free PMC article.
-
Mutual exacerbation of peroxisome proliferator-activated receptor γ coactivator 1α deregulation and α-synuclein oligomerization.Ann Neurol. 2015 Jan;77(1):15-32. doi: 10.1002/ana.24294. Epub 2014 Dec 19. Ann Neurol. 2015. PMID: 25363075 Free PMC article.
-
Posttranslational regulation of PGC-1α and its implication in cancer metabolism.Int J Cancer. 2019 Sep 15;145(6):1475-1483. doi: 10.1002/ijc.32253. Epub 2019 Mar 26. Int J Cancer. 2019. PMID: 30848477 Free PMC article. Review.
-
PGC-1α-mediated regulation of mitochondrial function and physiological implications.Appl Physiol Nutr Metab. 2020 Sep;45(9):927-936. doi: 10.1139/apnm-2020-0005. Epub 2020 Jun 9. Appl Physiol Nutr Metab. 2020. PMID: 32516539 Review.
Cited by
-
The contributory role of GSK3β in hypertension exacerbating atherosclerosis by regulating the OMA1/PGC1α pathway.Apoptosis. 2024 Oct 19. doi: 10.1007/s10495-024-02029-1. Online ahead of print. Apoptosis. 2024. PMID: 39427090
-
Dietary selenium intake and sarcopenia in American adults.Front Nutr. 2024 Sep 12;11:1449980. doi: 10.3389/fnut.2024.1449980. eCollection 2024. Front Nutr. 2024. PMID: 39328467 Free PMC article.
-
Emerging Multi-omic Approaches to the Molecular Diagnosis of Mitochondrial Disease and Available Strategies for Treatment and Prevention.Curr Genomics. 2024;25(5):358-379. doi: 10.2174/0113892029308327240612110334. Epub 2024 Jun 14. Curr Genomics. 2024. PMID: 39323625 Free PMC article. Review.
-
Is SIRT3 and Mitochondria a Reliable Target for Parkinson's Disease and Aging? A Narrative Review.Mol Neurobiol. 2024 Sep 17. doi: 10.1007/s12035-024-04486-w. Online ahead of print. Mol Neurobiol. 2024. PMID: 39287746 Review.
-
Organelle Communication with the Nucleus.Results Probl Cell Differ. 2024;73:3-23. doi: 10.1007/978-3-031-62036-2_1. Results Probl Cell Differ. 2024. PMID: 39242372 Review.
References
-
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science 2001;294:1866–70 - PubMed
-
- Francis GA, Fayard E, Picard F, Auwerx J. Nuclear receptors and the control of metabolism. Annu Rev Physiol 2003;65:261–311 - PubMed
-
- Spiegelman BM, Heinrich R. Biological control through regulated transcriptional coactivators. Cell 2004;119:157–67 - PubMed
-
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998;92:829–39 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

