The tuberin/mTOR pathway promotes apoptosis of tubular epithelial cells in diabetes
- PMID: 21289215
- PMCID: PMC3029899
- DOI: 10.1681/ASN.2010040352
The tuberin/mTOR pathway promotes apoptosis of tubular epithelial cells in diabetes
Abstract
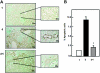
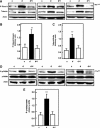
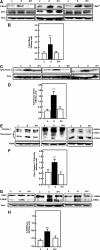
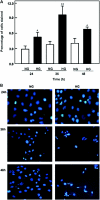
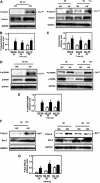
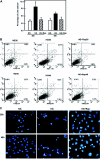
Apoptosis contributes to the development of diabetic nephropathy, but the mechanism by which high glucose (HG) induces apoptosis is not fully understood. Because the tuberin/mTOR pathway can modulate apoptosis, we studied the role of this pathway in apoptosis in type I diabetes and in cultured proximal tubular epithelial (PTE) cells exposed to HG. Compared with control rats, diabetic rats had more apoptotic cells in the kidney cortex. Induction of diabetes also increased phosphorylation of tuberin in association with mTOR activation (measured by p70S6K phosphorylation), inactivation of Bcl-2, increased cytosolic cytochrome c expression, activation of caspase 3, and cleavage of PARP; insulin treatment prevented these changes. In vitro, exposure of PTE cells to HG increased phosphorylation of tuberin and p70S6K, phosphorylation of Bcl-2, expression of cytosolic cytochrome c, and caspase 3 activity. High glucose induced translocation of the caspase substrate YY1 from the cytoplasm to the nucleus and enhanced cleavage of PARP. Pretreatment the cells with the mTOR inhibitor rapamycin reduced the number of apoptotic cells induced by HG and the downstream effects of mTOR activation noted above. Furthermore, gene silencing of tuberin with siRNA decreased cleavage of PARP. These data show that the tuberin/mTOR pathway promotes apoptosis of tubular epithelial cells in diabetes, mediated in part by cleavage of PARP by YY1.
Figures



Similar articles
-
Alterations in tubular epithelial cells in diabetic nephropathy.J Nephrol. 2013 Sep-Oct;26(5):865-9. doi: 10.5301/jn.5000287. Epub 2013 Aug 6. J Nephrol. 2013. PMID: 24052469
-
Tuberin inhibits production of the matrix protein fibronectin in diabetes.J Am Soc Nephrol. 2012 Oct;23(10):1652-62. doi: 10.1681/ASN.2012030285. Epub 2012 Aug 16. J Am Soc Nephrol. 2012. PMID: 22904348 Free PMC article.
-
The mTOR promotes oxidative stress-induced apoptosis of mesangial cells in diabetic nephropathy.Mol Cell Endocrinol. 2018 Sep 15;473:31-43. doi: 10.1016/j.mce.2017.12.012. Epub 2017 Dec 24. Mol Cell Endocrinol. 2018. PMID: 29277549
-
Mechanism of oxidative DNA damage in diabetes: tuberin inactivation and downregulation of DNA repair enzyme 8-oxo-7,8-dihydro-2'-deoxyguanosine-DNA glycosylase.Diabetes. 2008 Oct;57(10):2626-36. doi: 10.2337/db07-1579. Epub 2008 Jul 3. Diabetes. 2008. PMID: 18599524 Free PMC article.
-
Tumour suppressors hamartin and tuberin: intracellular signalling.Cell Signal. 2003 Aug;15(8):729-39. doi: 10.1016/s0898-6568(03)00040-8. Cell Signal. 2003. PMID: 12781866 Review.
Cited by
-
Protective role of p70S6K in intestinal ischemia/reperfusion injury in mice.PLoS One. 2012;7(7):e41584. doi: 10.1371/journal.pone.0041584. Epub 2012 Jul 27. PLoS One. 2012. PMID: 22848534 Free PMC article.
-
A new drug combination significantly reduces kidney tumor progression in kidney mouse model.Oncotarget. 2018 Aug 31;9(68):32900-32916. doi: 10.18632/oncotarget.26004. eCollection 2018 Aug 31. Oncotarget. 2018. PMID: 30250638 Free PMC article.
-
The tubular hypothesis of nephron filtration and diabetic kidney disease.Nat Rev Nephrol. 2020 Jun;16(6):317-336. doi: 10.1038/s41581-020-0256-y. Epub 2020 Mar 9. Nat Rev Nephrol. 2020. PMID: 32152499 Free PMC article. Review.
-
Galectin-1 is a new fibrosis protein in type 1 and type 2 diabetes.FASEB J. 2019 Jan;33(1):373-387. doi: 10.1096/fj.201800555RR. Epub 2018 Jul 5. FASEB J. 2019. PMID: 29975570 Free PMC article.
-
Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy.Int J Endocrinol. 2018 Apr 1;2018:1875870. doi: 10.1155/2018/1875870. eCollection 2018. Int J Endocrinol. 2018. PMID: 29808088 Free PMC article. Review.
References
-
- Verzola D, Bertolotto MB, Villaggio B, Ottonello L, Dallegri F, Berruti V, Gandolfo MT, Frumento G, Garibotto G, Defferrari G: Taurine prevents apoptosis induced by high ambient glucose in human tubule renal cells. J Investig Med 50: 443–451, 2002 - PubMed
-
- Allen AD, Harwood SM, Varagunam M, Raftery MJ, Yaqoob MM: High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J 17: 908–921, 2003 - PubMed
-
- Verzola D, Bertolotto MB, Villaggio B, Ottonello L, Dallegri F, Salvatore F, Berruti V, Gandolfo MT, Garibotto G, Defferrari G: Oxidative stress mediates apoptotic changes induced by hyperglycemia in human tubular kidney cells. J Am Soc Nephrol 15: S85–S87, 2004 - PubMed
-
- Park Sh, Choi HJ, Lee JH, Woo Ch, Kim JH, Han HJ: High glucose inhibits renal proximal tubule cell proliferation and involves PKC, oxidative stress and TGF-β1. Kidney Int 59: 1695–1705, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

