DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism
- PMID: 21285251
- PMCID: PMC3072762
- DOI: 10.1158/0008-5472.CAN-10-2738
DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism
Abstract
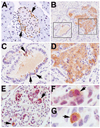
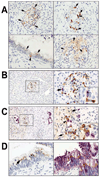
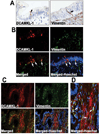
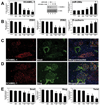
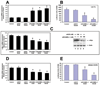
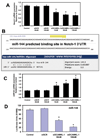
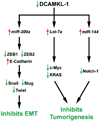
Pancreatic cancer is an exceptionally aggressive disease in great need of more effective therapeutic options. Epithelial-mesenchymal transition (EMT) plays a key role in cancer invasion and metastasis, and there is a gain of stem cell properties during EMT. Here we report increased expression of the putative pancreatic stem cell marker DCAMKL-1 in an established KRAS transgenic mouse model of pancreatic cancer and in human pancreatic adenocarcinoma. Colocalization of DCAMKL-1 with vimentin, a marker of mesenchymal lineage, along with 14-3-3 σ was observed within premalignant PanIN lesions that arise in the mouse model. siRNA-mediated knockdown of DCAMKL-1 in human pancreatic cancer cells induced microRNA miR-200a, an EMT inhibitor, along with downregulation of EMT-associated transcription factors ZEB1, ZEB2, Snail, Slug, and Twist. Furthermore, DCAMKL-1 knockdown resulted in downregulation of c-Myc and KRAS through a let-7a microRNA-dependent mechanism, and downregulation of Notch-1 through a miR-144 microRNA-dependent mechanism. These findings illustrate direct regulatory links between DCAMKL-1, microRNAs, and EMT in pancreatic cancer. Moreover, they demonstrate a functional role for DCAMKL-1 in pancreatic cancer. Together, our results rationalize DCAMKL-1 as a therapeutic target for eradicating pancreatic cancers.
© 2011 AACR.
Conflict of interest statement
Authors have no conflict of interest
Figures
Similar articles
-
XMD8-92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism.Cancer Lett. 2014 Aug 28;351(1):151-61. doi: 10.1016/j.canlet.2014.05.011. Epub 2014 May 28. Cancer Lett. 2014. PMID: 24880079
-
DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer.PLoS One. 2013 Sep 9;8(9):e73940. doi: 10.1371/journal.pone.0073940. eCollection 2013. PLoS One. 2013. PMID: 24040120 Free PMC article.
-
MiR-652 inhibits acidic microenvironment-induced epithelial-mesenchymal transition of pancreatic cancer cells by targeting ZEB1.Oncotarget. 2015 Nov 24;6(37):39661-75. doi: 10.18632/oncotarget.5350. Oncotarget. 2015. PMID: 26498682 Free PMC article.
-
F-box proteins: Keeping the epithelial-to-mesenchymal transition (EMT) in check.Semin Cancer Biol. 2016 Feb;36:71-9. doi: 10.1016/j.semcancer.2015.10.003. Epub 2015 Nov 11. Semin Cancer Biol. 2016. PMID: 26506454 Review.
-
Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer.Tumour Biol. 2014 Aug;35(8):7335-42. doi: 10.1007/s13277-014-2163-y. Epub 2014 Jun 2. Tumour Biol. 2014. PMID: 24880591 Review.
Cited by
-
Urine miRNAs: potential biomarkers for monitoring progression of early stages of diabetic nephropathy.Med Hypotheses. 2013 Aug;81(2):274-8. doi: 10.1016/j.mehy.2013.04.031. Epub 2013 May 14. Med Hypotheses. 2013. PMID: 23683774 Free PMC article. Clinical Trial.
-
Survival of Patients with Gastrointestinal Cancers Can Be Predicted by a Surrogate microRNA Signature for Cancer Stem-like Cells Marked by DCLK1 Kinase.Cancer Res. 2016 Jul 15;76(14):4090-9. doi: 10.1158/0008-5472.CAN-16-0029. Epub 2016 Jun 10. Cancer Res. 2016. PMID: 27287716 Free PMC article.
-
DCLK1 immunoreactivity in colorectal neoplasia.Clin Exp Gastroenterol. 2012;5:35-42. doi: 10.2147/CEG.S30281. Epub 2012 Apr 11. Clin Exp Gastroenterol. 2012. PMID: 22557932 Free PMC article.
-
Functional Significance and Therapeutic Potential of miR-15a Mimic in Pancreatic Ductal Adenocarcinoma.Mol Ther Nucleic Acids. 2020 Mar 6;19:228-239. doi: 10.1016/j.omtn.2019.11.010. Epub 2019 Nov 20. Mol Ther Nucleic Acids. 2020. PMID: 31846800 Free PMC article.
-
MicroRNA-195: a review of its role in cancers.Onco Targets Ther. 2018 Oct 17;11:7109-7123. doi: 10.2147/OTT.S183600. eCollection 2018. Onco Targets Ther. 2018. PMID: 30410367 Free PMC article. Review.
References
-
- Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45(7):823–830. - PubMed
-
- Diehn M, Clarke MF. Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst. 2006 Dec 20;98(24):1755–1757. - PubMed
-
- Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer research. 2007 Feb 1;67(3):1030–1037. - PubMed
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997 Jul;3(7):730–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 DK002822-05/DK/NIDDK NIH HHS/United States
- DK-065887/DK/NIDDK NIH HHS/United States
- CA-137482/CA/NCI NIH HHS/United States
- R01 DK062265/DK/NIDDK NIH HHS/United States
- R01 DK062265-07A2/DK/NIDDK NIH HHS/United States
- R21 CA137482/CA/NCI NIH HHS/United States
- R03 DK065887-04/DK/NIDDK NIH HHS/United States
- K08 DK002822-02/DK/NIDDK NIH HHS/United States
- R03 DK065887/DK/NIDDK NIH HHS/United States
- R03 DK065887-03/DK/NIDDK NIH HHS/United States
- R21 CA137482-01A2/CA/NCI NIH HHS/United States
- R03 DK065887-02/DK/NIDDK NIH HHS/United States
- K08 DK002822-04/DK/NIDDK NIH HHS/United States
- DK-002822/DK/NIDDK NIH HHS/United States
- R03 DK065887-01/DK/NIDDK NIH HHS/United States
- K08 DK002822/DK/NIDDK NIH HHS/United States
- R01 CA135559-03/CA/NCI NIH HHS/United States
- R01 CA109269/CA/NCI NIH HHS/United States
- R01 CA135559/CA/NCI NIH HHS/United States
- R01 DK062265-08/DK/NIDDK NIH HHS/United States
- K08 DK002822-03/DK/NIDDK NIH HHS/United States
- K08 DK002822-01A1/DK/NIDDK NIH HHS/United States
- R21 CA137482-02/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

