Time-resolved fluorescence resonance energy transfer (TR-FRET) to analyze the disruption of EGFR/HER2 dimers: a new method to evaluate the efficiency of targeted therapy using monoclonal antibodies
- PMID: 21282108
- PMCID: PMC3064190
- DOI: 10.1074/jbc.M111.223503
Time-resolved fluorescence resonance energy transfer (TR-FRET) to analyze the disruption of EGFR/HER2 dimers: a new method to evaluate the efficiency of targeted therapy using monoclonal antibodies
Abstract
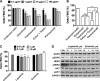
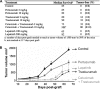
In oncology, simultaneous inhibition of epidermal growth factor receptor (EGFR) and HER2 by monoclonal antibodies (mAbs) is an efficient therapeutic strategy but the underlying mechanisms are not fully understood. Here, we describe a time-resolved fluorescence resonance energy transfer (TR-FRET) method to quantify EGFR/HER2 heterodimers on cell surface to shed some light on the mechanism of such therapies. First, we tested this antibody-based TR-FRET assay in NIH/3T3 cell lines that express EGFR and/or HER2 and in various tumor cell lines. Then, we used the antibody-based TR-FRET assay to evaluate in vitro the effect of different targeted therapies on EGFR/HER2 heterodimers in the ovarian carcinoma cell line SKOV-3. A simultaneous incubation with Cetuximab (anti-EGFR) and Trastuzumab (anti-HER2) disturbed EGFR/HER2 heterodimers resulting in a 72% reduction. Cetuximab, Trastuzumab or Pertuzumab (anti-HER2) alone induced a 48, 44, or 24% reduction, respectively. In contrast, the tyrosine kinase inhibitors Erlotinib and Lapatinib had very little effect on EGFR/HER2 dimers concentration. In vivo, the combination of Cetuximab and Trastuzumab showed a better therapeutic effect (median survival and percentage of tumor-free mice) than the single mAbs. These results suggest a correlation between the extent of the mAb-induced EGFR/HER2 heterodimer reduction and the efficacy of such mAbs in targeted therapies. In conclusion, quantifying EGFR/HER2 heterodimers using our antibody-based TR-FRET assay may represent a useful method to predict the efficacy and explain the mechanisms of action of therapeutic mAbs, in addition to other commonly used techniques that focus on antibody-dependent cellular cytotoxicity, phosphorylation, and cell proliferation.
Figures

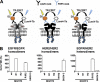

Similar articles
-
In pancreatic carcinoma, dual EGFR/HER2 targeting with cetuximab/trastuzumab is more effective than treatment with trastuzumab/erlotinib or lapatinib alone: implication of receptors' down-regulation and dimers' disruption.Neoplasia. 2012 Feb;14(2):121-30. doi: 10.1593/neo.111602. Neoplasia. 2012. PMID: 22431920 Free PMC article.
-
EGFR, HER2 and HER3 dimerization patterns guide targeted inhibition in two histotypes of esophageal cancer.Int J Cancer. 2014 Oct 1;135(7):1517-30. doi: 10.1002/ijc.28771. Epub 2014 Apr 2. Int J Cancer. 2014. PMID: 24510732
-
Combined use of anti-ErbB monoclonal antibodies and erlotinib enhances antibody-dependent cellular cytotoxicity of wild-type erlotinib-sensitive NSCLC cell lines.Mol Cancer. 2012 Dec 12;11:91. doi: 10.1186/1476-4598-11-91. Mol Cancer. 2012. PMID: 23234355 Free PMC article.
-
Epidermal growth factor receptor (EGFR) targeted therapies in non-small cell lung cancer (NSCLC).Rev Recent Clin Trials. 2006 Jan;1(1):1-13. doi: 10.2174/157488706775246157. Rev Recent Clin Trials. 2006. PMID: 18393776 Review.
-
The ErbB/HER family of protein-tyrosine kinases and cancer.Pharmacol Res. 2014 Jan;79:34-74. doi: 10.1016/j.phrs.2013.11.002. Epub 2013 Nov 20. Pharmacol Res. 2014. PMID: 24269963 Review.
Cited by
-
Synbindin in extracellular signal-regulated protein kinase spatial regulation and gastric cancer aggressiveness.J Natl Cancer Inst. 2013 Nov 20;105(22):1738-49. doi: 10.1093/jnci/djt271. Epub 2013 Oct 8. J Natl Cancer Inst. 2013. PMID: 24104608 Free PMC article.
-
Candida albicans stimulates formation of a multi-receptor complex that mediates epithelial cell invasion during oropharyngeal infection.PLoS Pathog. 2023 Aug 23;19(8):e1011579. doi: 10.1371/journal.ppat.1011579. eCollection 2023 Aug. PLoS Pathog. 2023. PMID: 37611070 Free PMC article.
-
Preclinical validation of AXL receptor as a target for antibody-based pancreatic cancer immunotherapy.Oncogene. 2014 Nov 20;33(47):5405-14. doi: 10.1038/onc.2013.487. Epub 2013 Nov 18. Oncogene. 2014. PMID: 24240689 Free PMC article.
-
Cetuximab response of lung cancer-derived EGF receptor mutants is associated with asymmetric dimerization.Cancer Res. 2013 Nov 15;73(22):6770-9. doi: 10.1158/0008-5472.CAN-13-1145. Epub 2013 Sep 24. Cancer Res. 2013. PMID: 24063894 Free PMC article.
-
In vivo multiplexed molecular imaging of esophageal cancer via spectral endoscopy of topically applied SERS nanoparticles.Biomed Opt Express. 2015 Sep 1;6(10):3714-23. doi: 10.1364/BOE.6.003714. eCollection 2015 Oct 1. Biomed Opt Express. 2015. PMID: 26504623 Free PMC article.
References
-
- Yarden Y., Sliwkowski M. X. (2001) Nat. Rev. Mol. Cell Biol. 2, 127–137 - PubMed
-
- Garrett T. P., McKern N. M., Lou M., Elleman T. C., Adams T. E., Lovrecz G. O., Zhu H. J., Walker F., Frenkel M. J., Hoyne P. A., Jorissen R. N., Nice E. C., Burgess A. W., Ward C. W. (2002) Cell 110, 763–773 - PubMed
-
- Hynes N. E., MacDonald G. (2009) Curr. Opin. Cell Biol. 21, 177–184 - PubMed
-
- Burgess A. W. (2008) Growth Factors 26, 263–274 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

