Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization
- PMID: 21281959
- PMCID: PMC3052379
- DOI: 10.1016/j.jaci.2010.12.1083
Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization
Abstract
Background: There are no treatments currently available for peanut allergy. Sublingual immunotherapy (SLIT) is a novel approach to the treatment of peanut allergy.
Objective: We sought to investigate the safety, clinical effectiveness, and immunologic changes with SLIT in children with peanut allergy.
Methods: In this double-blind, placebo-controlled study subjects underwent 6 months of dose escalation and 6 months of maintenance dosing followed by a double-blind, placebo-controlled food challenge.
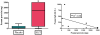
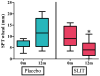
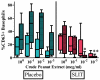
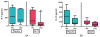
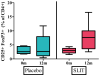
Results: Eighteen children aged 1 to 11 years completed 12 months of dosing and the food challenge. Dosing side effects were primarily oropharyngeal and uncommonly required treatment. During the double-blind, placebo-controlled food challenge, the treatment group safely ingested 20 times more peanut protein than the placebo group (median, 1,710 vs 85 mg; P = .011). Mechanistic studies demonstrated a decrease in skin prick test wheal size (P = .020) and decreased basophil responsiveness after stimulation with 10(-2) μg/mL (P = .009) and 10(-3) μg/mL (P = .009) of peanut. Peanut-specific IgE levels increased over the initial 4 months (P = .002) and then steadily decreased over the remaining 8 months (P = .003), whereas peanut-specific IgG4 levels increased during the 12 months (P = .014). Lastly, IL-5 levels decreased after 12 months (P = .015). No statistically significant changes were found in IL-13 levels, the percentage of regulatory T cells, or IL-10 and IFN-γ production.
Conclusion: Peanut SLIT is able to safely induce clinical desensitization in children with peanut allergy, with evidence of immunologic changes suggesting a significant change in the allergic response. Further study is required to determine whether continued peanut SLIT is able to induce long-term immune tolerance.
Copyright © 2011 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures
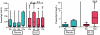
Similar articles
-
Long-term sublingual immunotherapy for peanut allergy in children: Clinical and immunologic evidence of desensitization.J Allergy Clin Immunol. 2019 Nov;144(5):1320-1326.e1. doi: 10.1016/j.jaci.2019.07.030. Epub 2019 Sep 4. J Allergy Clin Immunol. 2019. PMID: 31493887 Free PMC article. Clinical Trial.
-
A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy.J Allergy Clin Immunol. 2015 May;135(5):1275-82.e1-6. doi: 10.1016/j.jaci.2014.11.005. Epub 2014 Dec 18. J Allergy Clin Immunol. 2015. PMID: 25528358 Free PMC article. Clinical Trial.
-
A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response.J Allergy Clin Immunol. 2011 Mar;127(3):654-60. doi: 10.1016/j.jaci.2010.12.1111. J Allergy Clin Immunol. 2011. PMID: 21377034 Free PMC article. Clinical Trial.
-
Immunotherapy approaches for peanut allergy.Expert Rev Clin Immunol. 2020 Feb;16(2):167-174. doi: 10.1080/1744666X.2019.1708192. Epub 2020 Jan 12. Expert Rev Clin Immunol. 2020. PMID: 31928251 Review.
-
Update on peanut allergy: Prevention and immunotherapy.Allergy Asthma Proc. 2019 Jan 1;40(1):14-20. doi: 10.2500/aap.2019.40.4190. Allergy Asthma Proc. 2019. PMID: 30582491 Review.
Cited by
-
Biomarkers and mechanisms of tolerance induction in food allergic patients drive new therapeutic approaches.Front Immunol. 2022 Oct 3;13:972103. doi: 10.3389/fimmu.2022.972103. eCollection 2022. Front Immunol. 2022. PMID: 36263023 Free PMC article. Review.
-
Allergen-specific oral immunotherapy for peanut allergy.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD009014. doi: 10.1002/14651858.CD009014.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972130 Free PMC article. Review.
-
The future of food allergy therapeutics.Semin Immunopathol. 2012 Sep;34(5):703-14. doi: 10.1007/s00281-012-0319-7. Epub 2012 Jun 27. Semin Immunopathol. 2012. PMID: 22735939 Free PMC article. Review.
-
The Future of Sublingual Immunotherapy in the United States.Curr Allergy Asthma Rep. 2015 Aug;15(8):44. doi: 10.1007/s11882-015-0545-x. Curr Allergy Asthma Rep. 2015. PMID: 26149585 Review.
-
Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice.J Allergy Clin Immunol. 2013 Oct;132(4):881-8.e1-11. doi: 10.1016/j.jaci.2013.06.008. Epub 2013 Aug 1. J Allergy Clin Immunol. 2013. PMID: 23915716 Free PMC article.
References
-
- Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief. 2008 Oct 10;:1–8. - PubMed
-
- Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001 Jan;107(1):191–3. - PubMed
-
- Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007 Apr;119(4):1016–8. - PubMed
-
- Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010 Jun;125(6):1322–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

