Effects of EpCAM overexpression on human breast cancer cell lines
- PMID: 21281469
- PMCID: PMC3042418
- DOI: 10.1186/1471-2407-11-45
Effects of EpCAM overexpression on human breast cancer cell lines
Abstract
Background: Recently, EpCAM has attracted major interest as a target for antibody- and vaccine-based cancer immunotherapies. In breast cancer, the EpCAM antigen is overexpressed in 30-40% of all cases and this increased expression correlates with poor prognosis. The use of EpCAM-specific monoclonal antibodies is a promising treatment approach in these patients.
Methods: In order to explore molecular changes following EpCAM overexpression, we investigated changes of the transcriptome upon EpCAM gene expression in commercially available human breast cancer cells lines Hs578T and MDA-MB-231. To assess cell proliferation, a tetrazolium salt based assay was performed. A TCF/LEF Reporter Kit was used to measure the transcriptional activity of the Wnt/β-catenin pathway. To evaluate the accumulation of β-catenin in the nucleus, a subcellular fractionation assay was performed.
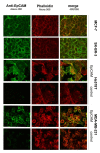
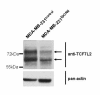
Results: For the first time we could show that expression profiling data of EpCAM transfected cell lines Hs578TEpCAM and MDA-MB-231EpCAM indicate an association of EpCAM overexpression with the downregulation of the Wnt signaling inhibitors SFRP1 and TCF7L2. Confirmation of increased Wnt signaling was provided by a TCF/LEF reporter kit and by the finding of the nuclear accumulation of ß-catenin for MDA-MB-231 EpCAM but not Hs578T EpCAM cells. In Hs578T cells, an increase of proliferation and chemosensitivity to Docetaxel was associated with EpCAM overexpression.
Conclusions: These data show a cell type dependent modification of Wnt signaling components after EpCAM overexpression in breast cancer cell lines, which results in marginal functional changes. Further investigations on the interaction of EpCAM with SFRP1 and TCF7L2 and on additional factors, which may be causal for changes upon EpCAM overexpression, will help to characterize unique molecular properties of EpCAM-positive breast cancer cells.
Figures

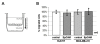
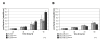



Similar articles
-
Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma.Cancer Res. 2007 Nov 15;67(22):10831-9. doi: 10.1158/0008-5472.CAN-07-0908. Cancer Res. 2007. PMID: 18006828
-
Phenotype-dependent effects of EpCAM expression on growth and invasion of human breast cancer cell lines.BMC Cancer. 2012 Oct 30;12:501. doi: 10.1186/1471-2407-12-501. BMC Cancer. 2012. PMID: 23110550 Free PMC article.
-
EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy.Cancer Res. 2004 Aug 15;64(16):5818-24. doi: 10.1158/0008-5472.CAN-04-0754. Cancer Res. 2004. PMID: 15313925
-
The emerging role of EpCAM in cancer and stem cell signaling.Cancer Res. 2009 Jul 15;69(14):5627-9. doi: 10.1158/0008-5472.CAN-09-0654. Epub 2009 Jul 7. Cancer Res. 2009. PMID: 19584271 Review.
-
A Wnt-ow of opportunity: targeting the Wnt/beta-catenin pathway in breast cancer.Curr Drug Targets. 2010 Sep;11(9):1074-88. doi: 10.2174/138945010792006780. Curr Drug Targets. 2010. PMID: 20545611 Review.
Cited by
-
Immunocapture of prostate cancer cells by use of anti-PSMA antibodies in microdevices.Biomed Microdevices. 2012 Apr;14(2):401-7. doi: 10.1007/s10544-011-9616-5. Biomed Microdevices. 2012. PMID: 22143878 Free PMC article.
-
Tumor necrosis factor-α-induced nuclear factor-kappaB activation in human cardiomyocytes is mediated by NADPH oxidase.J Physiol Biochem. 2014 Sep;70(3):769-79. doi: 10.1007/s13105-014-0345-0. Epub 2014 Jul 25. J Physiol Biochem. 2014. PMID: 25059721
-
Nuclear Ep-ICD accumulation predicts aggressive clinical course in early stage breast cancer patients.BMC Cancer. 2014 Sep 29;14:726. doi: 10.1186/1471-2407-14-726. BMC Cancer. 2014. PMID: 25265904 Free PMC article.
-
Complement inhibitor CSMD1 modulates epidermal growth factor receptor oncogenic signaling and sensitizes breast cancer cells to chemotherapy.J Exp Clin Cancer Res. 2021 Aug 17;40(1):258. doi: 10.1186/s13046-021-02042-1. J Exp Clin Cancer Res. 2021. PMID: 34404439 Free PMC article.
-
The association between Annexin A2 and epithelial cell adhesion molecule in breast cancer cells.Cancer Rep (Hoboken). 2022 May;5(5):e1498. doi: 10.1002/cnr2.1498. Epub 2021 Jul 9. Cancer Rep (Hoboken). 2022. PMID: 34240826 Free PMC article.
References
-
- Tandon AK, Clark GM, Chamness GC, McGuire WL. Association of the 323/A3 surface glycoprotein with tumor characteristics and behavior in human breast cancer. Cancer Res. 1990;50:3317–3321. - PubMed
-
- Spizzo G, Went P, Dirnhofer S, Obrist P, Simon R, Spichtin H, Maurer R, Metzger U, Von Castelberg B, Bart R. et al.High Ep-CAM Expression is Associated with Poor Prognosis in Node-positive Breast Cancer. Breast Cancer Res Treat. 2004;86:207–213. doi: 10.1023/B:BREA.0000036787.59816.01. - DOI - PubMed
-
- Schmidt M, Hasenclever D, Schaeffer M, Boehm D, Cotarelo C, Steiner E, Lebrecht A, Siggelkow W, Weikel W, Schiffer-Petry I. et al.Prognostic effect of epithelial cell adhesion molecule overexpression in untreated node-negative breast cancer. Clin Cancer Res. 2008;14:5849–5855. doi: 10.1158/1078-0432.CCR-08-0669. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

