Habenular α5 nicotinic receptor subunit signalling controls nicotine intake
- PMID: 21278726
- PMCID: PMC3079537
- DOI: 10.1038/nature09797
Habenular α5 nicotinic receptor subunit signalling controls nicotine intake
Abstract
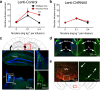
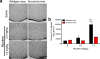
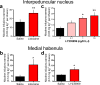
Genetic variation in CHRNA5, the gene encoding the α5 nicotinic acetylcholine receptor subunit, increases vulnerability to tobacco addiction and lung cancer, but the underlying mechanisms are unknown. Here we report markedly increased nicotine intake in mice with a null mutation in Chrna5. This effect was 'rescued' in knockout mice by re-expressing α5 subunits in the medial habenula (MHb), and recapitulated in rats through α5 subunit knockdown in MHb. Remarkably, α5 subunit knockdown in MHb did not alter the rewarding effects of nicotine but abolished the inhibitory effects of higher nicotine doses on brain reward systems. The MHb extends projections almost exclusively to the interpeduncular nucleus (IPN). We found diminished IPN activation in response to nicotine in α5 knockout mice. Further, disruption of IPN signalling increased nicotine intake in rats. Our findings indicate that nicotine activates the habenulo-interpeduncular pathway through α5-containing nAChRs, triggering an inhibitory motivational signal that acts to limit nicotine intake.
Figures


Similar articles
-
α3* Nicotinic Acetylcholine Receptors in the Habenula-Interpeduncular Nucleus Circuit Regulate Nicotine Intake.J Neurosci. 2021 Feb 24;41(8):1779-1787. doi: 10.1523/JNEUROSCI.0127-19.2020. Epub 2020 Dec 30. J Neurosci. 2021. PMID: 33380469 Free PMC article.
-
Retrograde inhibition by a specific subset of interpeduncular α5 nicotinic neurons regulates nicotine preference.Proc Natl Acad Sci U S A. 2017 Dec 5;114(49):13012-13017. doi: 10.1073/pnas.1717506114. Epub 2017 Nov 20. Proc Natl Acad Sci U S A. 2017. PMID: 29158387 Free PMC article.
-
β4-Nicotinic Receptors Are Critically Involved in Reward-Related Behaviors and Self-Regulation of Nicotine Reinforcement.J Neurosci. 2020 Apr 22;40(17):3465-3477. doi: 10.1523/JNEUROSCI.0356-19.2020. Epub 2020 Mar 17. J Neurosci. 2020. PMID: 32184221 Free PMC article.
-
The habenulo-interpeduncular pathway in nicotine aversion and withdrawal.Neuropharmacology. 2015 Sep;96(Pt B):213-22. doi: 10.1016/j.neuropharm.2014.11.019. Epub 2014 Dec 2. Neuropharmacology. 2015. PMID: 25476971 Free PMC article. Review.
-
Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior.Biochem Pharmacol. 2011 Oct 15;82(8):984-95. doi: 10.1016/j.bcp.2011.06.026. Epub 2011 Jun 29. Biochem Pharmacol. 2011. PMID: 21740894 Free PMC article. Review.
Cited by
-
Multidimensional Intersection of Nicotine, Gene Expression, and Behavior.Front Behav Neurosci. 2021 Mar 22;15:649129. doi: 10.3389/fnbeh.2021.649129. eCollection 2021. Front Behav Neurosci. 2021. PMID: 33828466 Free PMC article.
-
Nicotine withdrawal.Curr Top Behav Neurosci. 2015;24:99-123. doi: 10.1007/978-3-319-13482-6_4. Curr Top Behav Neurosci. 2015. PMID: 25638335 Free PMC article. Review.
-
Striatal α5 nicotinic receptor subunit regulates dopamine transmission in dorsal striatum.J Neurosci. 2012 Feb 15;32(7):2352-6. doi: 10.1523/JNEUROSCI.4985-11.2012. J Neurosci. 2012. PMID: 22396410 Free PMC article.
-
Genetic variation (CHRNA5), medication (combination nicotine replacement therapy vs. varenicline), and smoking cessation.Drug Alcohol Depend. 2015 Sep 1;154:278-82. doi: 10.1016/j.drugalcdep.2015.06.022. Epub 2015 Jun 23. Drug Alcohol Depend. 2015. PMID: 26142345 Free PMC article. Clinical Trial.
-
Variation in the α 5 nicotinic acetylcholine receptor subunit gene predicts cigarette smoking intensity as a function of nicotine content.Pharmacogenomics J. 2014 Feb;14(1):70-6. doi: 10.1038/tpj.2012.50. Epub 2013 Jan 29. Pharmacogenomics J. 2014. PMID: 23358500 Free PMC article.
References
-
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. - PubMed
-
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. - PubMed
-
- Le Novere N, Corringer PJ, Changeux JP. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol. 2002;53:447–456. - PubMed
-
- Picciotto MR, et al. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. - PubMed
-
- Tapper AR, et al. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

