A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer
- PMID: 21276608
- PMCID: PMC4426879
- DOI: 10.1016/j.ygyno.2010.12.362
A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer
Abstract
Objective: This phase II, multicenter, single-arm, two-stage study in platinum-resistant, advanced epithelial ovarian or primary peritoneal cancer evaluated the efficacy, safety, and tolerability of weekly single-agent volociximab. Pharmacokinetic/pharmacodynamic (PK/PD) studies were also performed.
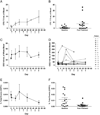
Methods: Sixteen patients were enrolled in Stage 1. Volociximab was administered at 15mg/kg IV qwk until progression of disease or drug intolerability. Tumor response was assessed every 8weeks. Serum samples for PK or whole blood for the evaluation of circulating tumor cells, endothelial cells, and endothelial progenitor cells were obtained on Days 1, 8, 15, 29, and 50. Ascites from one patient was collected for volociximab concentration analysis. Archived tumor tissue was analyzed by immunohistochemistry (IHC) for α5 integrin expression.
Results: Safety data are available on all 16 patients; 14 were evaluable for efficacy. One patient had stable disease at 8weeks. The remaining 13 progressed on treatment. Twelve patients (75%) experienced study-related adverse events (AEs); the most common (≥20%) were headache and fatigue. Three patients experienced possible study-related serious AEs (SAEs): reversible posterior leukoencephalopathy syndrome, pulmonary embolism, and hyponatremia. Peak serum concentrations of volociximab increased 2-3 fold from Day 1 to Day 50. Clinically relevant trough levels were achieved (>150μg/mL). IHC analysis of archived tumor sections showed low-to-moderate expression of α5 integrin on all ovarian cancer tissue evaluated.
Conclusion: Despite insufficient clinical activity in this refractory patient population to continue the study, weekly volociximab was well tolerated, and the gained understanding of the mechanism of action of volociximab will inform future development efforts.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
Katherine M. Bell-McGuinn: None.
Carolyn M. Matthews: None.
Minal Barve: None.
Lucy Gilbert: None.
Russell J. Schilder: None.
Figures


Similar articles
-
Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study.Gynecol Oncol. 2011 Oct;123(1):19-26. doi: 10.1016/j.ygyno.2011.06.022. Epub 2011 Jul 12. Gynecol Oncol. 2011. PMID: 21752435 Free PMC article. Clinical Trial.
-
Phase Ib safety and pharmacokinetic study of volociximab, an anti-α5β1 integrin antibody, in combination with carboplatin and paclitaxel in advanced non-small-cell lung cancer.Ann Oncol. 2013 Jan;24(1):90-6. doi: 10.1093/annonc/mds281. Epub 2012 Aug 16. Ann Oncol. 2013. PMID: 22904239 Clinical Trial.
-
A phase II trial with anti-Lewis-Y monoclonal antibody (hu3S193) for the treatment of platinum resistant/refractory ovarian, fallopian tube and primary peritoneal carcinoma.Gynecol Oncol. 2015 Aug;138(2):272-7. doi: 10.1016/j.ygyno.2015.05.023. Epub 2015 May 27. Gynecol Oncol. 2015. PMID: 26026738 Clinical Trial.
-
Volociximab in cancer.Expert Opin Biol Ther. 2012 Feb;12(2):251-7. doi: 10.1517/14712598.2012.646985. Epub 2011 Dec 22. Expert Opin Biol Ther. 2012. PMID: 22192080 Review.
-
Role of Circulating Biomarkers in Platinum-Resistant Ovarian Cancer.Int J Mol Sci. 2021 Dec 20;22(24):13650. doi: 10.3390/ijms222413650. Int J Mol Sci. 2021. PMID: 34948446 Free PMC article. Review.
Cited by
-
Lunasin sensitivity in non-small cell lung cancer cells is linked to suppression of integrin signaling and changes in histone acetylation.Int J Mol Sci. 2014 Dec 18;15(12):23705-24. doi: 10.3390/ijms151223705. Int J Mol Sci. 2014. PMID: 25530619 Free PMC article.
-
uPAR: a modulator of VEGF-induced angiogenesis.Cell Adh Migr. 2013 Jan-Feb;7(1):23-6. doi: 10.4161/cam.22124. Epub 2012 Oct 17. Cell Adh Migr. 2013. PMID: 23076213 Free PMC article.
-
Bone Metastasis from Renal Cell Carcinoma.Int J Mol Sci. 2016 Jun 22;17(6):987. doi: 10.3390/ijms17060987. Int J Mol Sci. 2016. PMID: 27338367 Free PMC article. Review.
-
Therapeutic targets and new directions for antibodies developed for ovarian cancer.MAbs. 2016 Nov/Dec;8(8):1437-1455. doi: 10.1080/19420862.2016.1219005. Epub 2016 Aug 5. MAbs. 2016. PMID: 27494775 Free PMC article. Review.
-
Angiogenesis in cancer: Anti-VEGF escape mechanisms.Transl Lung Cancer Res. 2012 Mar;1(1):14-25. doi: 10.3978/j.issn.2218-6751.2011.11.02. Transl Lung Cancer Res. 2012. PMID: 25806151 Free PMC article. Review.
References
-
- Francis SE, Goh KL, Hodivala-Dilke K, et al. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22:927–933. - PubMed
-
- Ramakrishnan V, Bhaskar V, Law DA, et al. Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J Exp Ther Oncol. 2006;5:273–286. - PubMed
-
- Markowska J, Szala S. Inhibitors of angiogenesis in therapy of ovarian cancers. Eur J Gynaecol Oncol. 2004;25:562–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

