Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation
- PMID: 21266776
- PMCID: PMC3026720
- DOI: 10.1172/JCI42974
Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation
Abstract
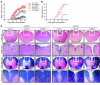
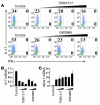
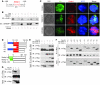
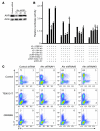
Th17 cells are a subset of CD4+ T cells with an important role in clearing certain bacterial and fungal pathogens. However, they have also been implicated in autoimmune diseases such as multiple sclerosis. Exposure of naive CD4+ T cells to IL-6 and TGF-β leads to Th17 cell differentiation through a process in which many proteins have been implicated. We report here that ectopic expression of liver X receptor (LXR) inhibits Th17 polarization of mouse CD4+ T cells, while LXR deficiency promotes Th17 differentiation in vitro. LXR activation in mice ameliorated disease in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis, whereas LXR deficiency exacerbated disease. Further analysis revealed that Srebp-1, which is encoded by an LXR target gene, mediated the suppression of Th17 differentiation by binding to the E-box element on the Il17 promoter, physically interacting with aryl hydrocarbon receptor (Ahr) and inhibiting Ahr-controlled Il17 transcription. The putative active site (PAS) domain of Ahr and the N-terminal acidic region of Srebp-1 were essential for this interaction. Additional analyses suggested that similar LXR-dependent mechanisms were operational during human Th17 differentiation in vitro. This study reports what we believe to be a novel signaling pathway underlying LXR-mediated regulation of Th17 cell differentiation and autoimmunity.
Figures





Comment in
-
Nuclear receptors take center stage in Th17 cell-mediated autoimmunity.J Clin Invest. 2011 Feb;121(2):519-21. doi: 10.1172/JCI45939. Epub 2011 Jan 25. J Clin Invest. 2011. PMID: 21266768 Free PMC article.
Similar articles
-
Nuclear receptors take center stage in Th17 cell-mediated autoimmunity.J Clin Invest. 2011 Feb;121(2):519-21. doi: 10.1172/JCI45939. Epub 2011 Jan 25. J Clin Invest. 2011. PMID: 21266768 Free PMC article.
-
TGFβ1 signaling sustains aryl hydrocarbon receptor (AHR) expression and restrains the pathogenic potential of TH17 cells by an AHR-independent mechanism.Cell Death Dis. 2018 Nov 13;9(11):1130. doi: 10.1038/s41419-018-1107-7. Cell Death Dis. 2018. PMID: 30425241 Free PMC article.
-
Liver X receptor agonist regulation of Th17 lymphocyte function in autoimmunity.J Leukoc Biol. 2009 Aug;86(2):401-9. doi: 10.1189/jlb.1008600. J Leukoc Biol. 2009. PMID: 19406833 Free PMC article.
-
IL-17 and related cytokines involved in the pathology and immunotherapy of multiple sclerosis: Current and future developments.Cytokine Growth Factor Rev. 2014 Aug;25(4):403-13. doi: 10.1016/j.cytogfr.2014.07.013. Epub 2014 Jul 29. Cytokine Growth Factor Rev. 2014. PMID: 25153998 Review.
-
The pathogenicity of Th17 cells in autoimmune diseases.Semin Immunopathol. 2019 May;41(3):283-297. doi: 10.1007/s00281-019-00733-8. Epub 2019 Mar 19. Semin Immunopathol. 2019. PMID: 30891627 Review.
Cited by
-
Metabolic control of the Treg/Th17 axis.Immunol Rev. 2013 Mar;252(1):52-77. doi: 10.1111/imr.12029. Immunol Rev. 2013. PMID: 23405895 Free PMC article. Review.
-
Metabolic checkpoints in activated T cells.Nat Immunol. 2012 Oct;13(10):907-15. doi: 10.1038/ni.2386. Epub 2012 Sep 18. Nat Immunol. 2012. PMID: 22990888 Review.
-
mTOR, metabolism, and the regulation of T-cell differentiation and function.Immunol Rev. 2012 Sep;249(1):43-58. doi: 10.1111/j.1600-065X.2012.01152.x. Immunol Rev. 2012. PMID: 22889214 Free PMC article. Review.
-
LXR-mediated inhibition of CD4+ T helper cells.PLoS One. 2012;7(9):e46615. doi: 10.1371/journal.pone.0046615. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23029557 Free PMC article.
-
Liver X Receptor Alpha Is Important in Maintaining Blood-Brain Barrier Function.Front Immunol. 2019 Jul 31;10:1811. doi: 10.3389/fimmu.2019.01811. eCollection 2019. Front Immunol. 2019. PMID: 31417573 Free PMC article.
References
-
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. - PubMed
-
- Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

