AaCAT1 of the yellow fever mosquito, Aedes aegypti: a novel histidine-specific amino acid transporter from the SLC7 family
- PMID: 21262963
- PMCID: PMC3060531
- DOI: 10.1074/jbc.M110.179739
AaCAT1 of the yellow fever mosquito, Aedes aegypti: a novel histidine-specific amino acid transporter from the SLC7 family
Abstract
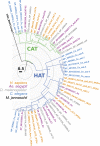
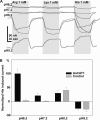
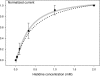
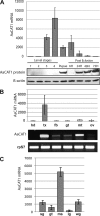
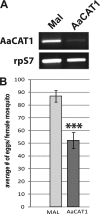
Insect yolk protein precursor gene expression is regulated by nutritional and endocrine signals. A surge of amino acids in the hemolymph of blood-fed female mosquitoes activates a nutrient signaling system in the fat bodies, which subsequently derepresses yolk protein precursor genes and makes them responsive to activation by steroid hormones. Orphan transporters of the SLC7 family were identified as essential upstream components of the nutrient signaling system in the fat body of fruit flies and the yellow fever mosquito, Aedes aegypti. However, the transport function of these proteins was unknown. We report expression and functional characterization of AaCAT1, cloned from the fat body of A. aegypti. Expression of AaCAT1 transcript and protein undergoes dynamic changes during postembryonic development of the mosquito. Transcript expression was especially high in the third and fourth larval stages; however, the AaCAT1 protein was detected only in pupa and adult stages. Functional expression and analysis of AaCAT1 in Xenopus oocytes revealed that it acts as a sodium-independent cationic amino acid transporter, with unique selectivity to L-histidine at neutral pH (K(0.5)(L-His) = 0.34 ± 0.07 mM, pH 7.2). Acidification to pH 6.2 dramatically increases AaCAT1-specific His(+)-induced current. RNAi-mediated silencing of AaCAT1 reduces egg yield of subsequent ovipositions. Our data show that AaCAT1 has notable differences in its transport mechanism when compared with related mammalian cationic amino acid transporters. It may execute histidine-specific transport and signaling in mosquito tissues.
Figures



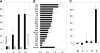
Similar articles
-
Substrate specificity and transport mechanism of amino-acid transceptor Slimfast from Aedes aegypti.Nat Commun. 2015 Oct 9;6:8546. doi: 10.1038/ncomms9546. Nat Commun. 2015. PMID: 26449545 Free PMC article.
-
SLC7 amino acid transporters of the yellow fever mosquito Aedes aegypti and their role in fat body TOR signaling and reproduction.J Insect Physiol. 2012 Apr;58(4):513-22. doi: 10.1016/j.jinsphys.2012.01.005. Epub 2012 Jan 15. J Insect Physiol. 2012. PMID: 22266018 Free PMC article.
-
NHE8 is an intracellular cation/H+ exchanger in renal tubules of the yellow fever mosquito Aedes aegypti.Am J Physiol Renal Physiol. 2009 Apr;296(4):F730-50. doi: 10.1152/ajprenal.90564.2008. Epub 2009 Feb 4. Am J Physiol Renal Physiol. 2009. PMID: 19193723 Free PMC article.
-
Molecular characteristics of mammalian and insect amino acid transporters: implications for amino acid homeostasis.J Exp Biol. 1997 Jan;200(Pt 2):269-86. doi: 10.1242/jeb.200.2.269. J Exp Biol. 1997. PMID: 9050235 Review.
-
NHE(VNAT): an H+ V-ATPase electrically coupled to a Na+:nutrient amino acid transporter (NAT) forms an Na+/H+ exchanger (NHE).J Exp Biol. 2009 Feb;212(Pt 3):347-57. doi: 10.1242/jeb.026047. J Exp Biol. 2009. PMID: 19151209 Free PMC article. Review.
Cited by
-
Transcriptome Sequencing Analysis and Functional Identification of Sex Differentiation Genes from the Mosquito Parasitic Nematode, Romanomermis wuchangensis.PLoS One. 2016 Sep 23;11(9):e0163127. doi: 10.1371/journal.pone.0163127. eCollection 2016. PLoS One. 2016. PMID: 27662191 Free PMC article.
-
Two of the three Transformer-2 genes are required for ovarian development in Aedes albopictus.Insect Biochem Mol Biol. 2019 Jun;109:92-105. doi: 10.1016/j.ibmb.2019.03.008. Epub 2019 Mar 23. Insect Biochem Mol Biol. 2019. PMID: 30914323 Free PMC article.
-
Substrate specificity and transport mechanism of amino-acid transceptor Slimfast from Aedes aegypti.Nat Commun. 2015 Oct 9;6:8546. doi: 10.1038/ncomms9546. Nat Commun. 2015. PMID: 26449545 Free PMC article.
-
Nerveless and gutsy: intestinal nutrient sensing from invertebrates to humans.Semin Cell Dev Biol. 2012 Aug;23(6):614-20. doi: 10.1016/j.semcdb.2012.01.002. Epub 2012 Jan 11. Semin Cell Dev Biol. 2012. PMID: 22248674 Free PMC article. Review.
-
Functional characterization of aquaporins and aquaglyceroporins of the yellow fever mosquito, Aedes aegypti.Sci Rep. 2015 Jan 15;5:7795. doi: 10.1038/srep07795. Sci Rep. 2015. PMID: 25589229 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

