Loss of the Birt-Hogg-Dubé tumor suppressor results in apoptotic resistance due to aberrant TGFβ-mediated transcription
- PMID: 21258407
- PMCID: PMC3109270
- DOI: 10.1038/onc.2010.628
Loss of the Birt-Hogg-Dubé tumor suppressor results in apoptotic resistance due to aberrant TGFβ-mediated transcription
Abstract
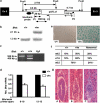
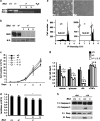
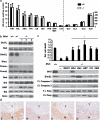
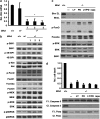
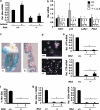
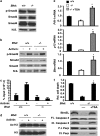
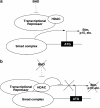
Birt-Hogg-Dubé (BHD) syndrome is an inherited cancer susceptibility disease characterized by skin and kidney tumors, as well as cystic lung disease, which results from loss-of-function mutations in the BHD gene. BHD is also inactivated in a significant fraction of patients with sporadic renal cancers and idiopathic cystic lung disease, and little is known about its mode of action. To investigate the molecular and cellular basis of BHD tumor suppressor activity, we generated mutant Bhd mice and embryonic stem cell lines. BHD-deficient cells exhibited defects in cell-intrinsic apoptosis that correlated with reduced expression of the BH3-only protein Bim, which was similarly observed in all human and murine BHD-related tumors examined. We further demonstrate that Bim deficiency in Bhd(-/-) cells is not a consequence of elevated mTOR or ERK activity, but results instead from reduced Bim transcription associated with a general loss of TGFβ-mediated transcription and chromatin modifications. In aggregate, this work identifies a specific tumor suppressive mechanism for BHD in regulating TGFβ-dependent transcription and apoptosis, which has implications for the development of targeted therapies.
Figures
Similar articles
-
Birt-Hogg-Dubé syndrome: Clinical and molecular aspects of recently identified kidney cancer syndrome.Int J Urol. 2016 Mar;23(3):204-10. doi: 10.1111/iju.13015. Epub 2015 Nov 25. Int J Urol. 2016. PMID: 26608100 Review.
-
Pulmonary cysts of Birt-Hogg-Dubé syndrome: a clinicopathologic and immunohistochemical study of 9 families.Am J Surg Pathol. 2012 Apr;36(4):589-600. doi: 10.1097/PAS.0b013e3182475240. Am J Surg Pathol. 2012. PMID: 22441547
-
Kidney-targeted Birt-Hogg-Dube gene inactivation in a mouse model: Erk1/2 and Akt-mTOR activation, cell hyperproliferation, and polycystic kidneys.J Natl Cancer Inst. 2008 Jan 16;100(2):140-54. doi: 10.1093/jnci/djm288. Epub 2008 Jan 8. J Natl Cancer Inst. 2008. PMID: 18182616 Free PMC article.
-
Birt-Hogg-Dubé syndrome: from gene discovery to molecularly targeted therapies.Fam Cancer. 2013 Sep;12(3):357-64. doi: 10.1007/s10689-012-9574-y. Fam Cancer. 2013. PMID: 23108783 Free PMC article.
-
Pathology of Birt-Hogg-Dubé syndrome: A special reference of pulmonary manifestations in a Japanese population with a comprehensive analysis and review.Pathol Int. 2019 Jan;69(1):1-12. doi: 10.1111/pin.12752. Epub 2019 Jan 11. Pathol Int. 2019. PMID: 30632664 Review.
Cited by
-
Over-production of nitric oxide by oxidative stress-induced activation of the TGF-β1/PI3K/Akt pathway in mesangial cells cultured in high glucose.Acta Pharmacol Sin. 2013 Apr;34(4):507-14. doi: 10.1038/aps.2012.207. Epub 2013 Mar 25. Acta Pharmacol Sin. 2013. PMID: 23524565 Free PMC article.
-
Lymphoplasmacytic lymphoma in a patient with Birt-Hogg-Dubé syndrome.Int J Hematol. 2020 Dec;112(6):864-870. doi: 10.1007/s12185-020-02970-2. Epub 2020 Aug 12. Int J Hematol. 2020. PMID: 32789566
-
Loss of FLCN inhibits canonical WNT signaling via TFE3.Hum Mol Genet. 2019 Oct 1;28(19):3270-3281. doi: 10.1093/hmg/ddz158. Hum Mol Genet. 2019. PMID: 31272105 Free PMC article.
-
Folliculin regulates ampk-dependent autophagy and metabolic stress survival.PLoS Genet. 2014 Apr 24;10(4):e1004273. doi: 10.1371/journal.pgen.1004273. eCollection 2014 Apr. PLoS Genet. 2014. PMID: 24763318 Free PMC article.
-
Regulation of Bim in Health and Disease.Oncotarget. 2015 Sep 15;6(27):23058-134. doi: 10.18632/oncotarget.5492. Oncotarget. 2015. PMID: 26405162 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

