The amazing osteocyte
- PMID: 21254230
- PMCID: PMC3179345
- DOI: 10.1002/jbmr.320
The amazing osteocyte
Abstract
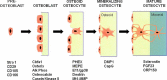
The last decade has provided a virtual explosion of data on the molecular biology and function of osteocytes. Far from being the "passive placeholder in bone," this cell has been found to have numerous functions, such as acting as an orchestrator of bone remodeling through regulation of both osteoclast and osteoblast activity and also functioning as an endocrine cell. The osteocyte is a source of soluble factors not only to target cells on the bone surface but also to target distant organs, such as kidney, muscle, and other tissues. This cell plays a role in both phosphate metabolism and calcium availability and can remodel its perilacunar matrix. Osteocytes compose 90% to 95% of all bone cells in adult bone and are the longest lived bone cell, up to decades within their mineralized environment. As we age, these cells die, leaving behind empty lacunae that frequently micropetrose. In aged bone such as osteonecrotic bone, empty lacunae are associated with reduced remodeling. Inflammatory factors such as tumor necrosis factor and glucocorticoids used to treat inflammatory disease induce osteocyte cell death, but by different mechanisms with potentially different outcomes. Therefore, healthy, viable osteocytes are necessary for proper functionality of bone and other organs.
Copyright © 2011 American Society for Bone and Mineral Research.
Figures

Similar articles
-
Physiological and pathological osteocytic osteolysis.J Musculoskelet Neuronal Interact. 2018 Sep 1;18(3):292-303. J Musculoskelet Neuronal Interact. 2018. PMID: 30179206 Free PMC article. Review.
-
The osteocyte: A multifunctional cell within the bone.Ann Anat. 2020 Jan;227:151422. doi: 10.1016/j.aanat.2019.151422. Epub 2019 Sep 26. Ann Anat. 2020. PMID: 31563568 Review.
-
Human primary osteocyte differentiation in a 3D culture system.J Bone Miner Res. 2009 Nov;24(11):1927-35. doi: 10.1359/jbmr.090517. J Bone Miner Res. 2009. PMID: 19419324
-
Vitamin D regulates osteocyte survival and perilacunar remodeling in human and murine bone.Bone. 2017 Oct;103:78-87. doi: 10.1016/j.bone.2017.06.022. Epub 2017 Jun 27. Bone. 2017. PMID: 28666969
-
A Novel Osteogenic Cell Line That Differentiates Into GFP-Tagged Osteocytes and Forms Mineral With a Bone-Like Lacunocanalicular Structure.J Bone Miner Res. 2019 Jun;34(6):979-995. doi: 10.1002/jbmr.3720. Epub 2019 Jun 7. J Bone Miner Res. 2019. PMID: 30882939 Free PMC article.
Cited by
-
The role of osteocytes in targeted bone remodeling: a mathematical model.PLoS One. 2013 May 22;8(5):e63884. doi: 10.1371/journal.pone.0063884. Print 2013. PLoS One. 2013. PMID: 23717504 Free PMC article.
-
Extracellular Vesicle-Mediated Bone Remodeling and Bone Metastasis: Implications in Prostate Cancer.Subcell Biochem. 2021;97:297-361. doi: 10.1007/978-3-030-67171-6_12. Subcell Biochem. 2021. PMID: 33779922 Review.
-
Serum sclerostin levels decline in post-menopausal women with osteoporosis following treatment with intermittent parathyroid hormone.J Endocrinol Invest. 2012 Oct;35(9):866-8. doi: 10.3275/8522. Epub 2012 Jul 24. J Endocrinol Invest. 2012. PMID: 22842667 Clinical Trial.
-
Nuclear factor erythroid 2-related factor 2 (Nrf2) deficiency causes age-dependent progression of female osteoporosis.BMC Musculoskelet Disord. 2022 Nov 25;23(1):1015. doi: 10.1186/s12891-022-05942-1. BMC Musculoskelet Disord. 2022. PMID: 36434613 Free PMC article.
-
Lacunar-canalicular bone remodeling: Impacts on bone quality and tools for assessment.Bone. 2021 Feb;143:115663. doi: 10.1016/j.bone.2020.115663. Epub 2020 Sep 26. Bone. 2021. PMID: 32987198 Free PMC article. Review.
References
-
- Recklinghausen FV. Untersuchungen uber Rachitis und Osteomalacia. Jena: Gustav Fischer; 1920.
-
- Belanger LF, Robichon J. Parathormone-induced osteolysis in dogs: a microradiographic and alpharadiographic survey. J Bone Joint Surg Am. 1964;46:1008–1012. - PubMed
-
- Belanger LF. Osteocytic osteolysis. Calcif Tissue Res. 1969;4:1–12. - PubMed
-
- Baylink DJ, Wergedal JE. Bone formation by osteocytes. Am J Physiol. 1971;221:669–678. - PubMed
-
- Pead MJ, Suswillo R, Skerry TM, Vedi S. Lanyon LE. Increased 3H-uridine levels in osteocytes following a. single short period of dynamic bone loading in vivo. Calcif Tissue Int. 1988;43:92–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

