Threonine 48 in the BIR domain of survivin is critical to its mitotic and anti-apoptotic activities and can be phosphorylated by CK2 in vitro
- PMID: 21252625
- PMCID: PMC3115020
- DOI: 10.4161/cc.10.3.14758
Threonine 48 in the BIR domain of survivin is critical to its mitotic and anti-apoptotic activities and can be phosphorylated by CK2 in vitro
Abstract
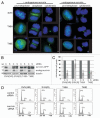
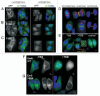
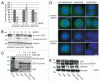
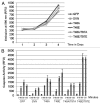
In this study we report that the protein kinase CK2 phosphorylates survivin specifically on threonine 48 (T48) within its BIR domain, and that T48 is critical to both the mitotic and anti-apoptotic roles of survivin. Interestingly, during mitosis T48 mutants localise normally, but are unable to support cell growth when endogenous survivin is removed by siRNA. In addition, while overexpression of survivin normally confers inhibition of TRAIL-mediated apoptosis, this protection is abolished by mutation of T48. Furthermore in interphase cells depletion of endogenous survivin causes redistribution of T48 mutants from the cytoplasm to the nucleus and treatment of cells expressing survivin-GFP with the CK2 inhibitor TBB phenocopies this nuclear redistribution. Finally, we show T48 mutants have increased affinity for borealin, and that this association and cell proliferation can be restored by introduction of a second mutation at T97. To our knowledge these data are the first to identify T48 as a key regulatory site on survivin, and CK2 as a mediator of its mitotic and anti-apoptotic functions.
Figures


Comment in
-
An emerging function of survivin's BIR domain phosphorylation in the control of cell division and cell death.Cell Cycle. 2011 Mar 15;10(6):876-7. Epub 2011 Mar 15. Cell Cycle. 2011. PMID: 21350336 No abstract available.
Similar articles
-
Separating the anti-apoptotic and mitotic roles of survivin.J Biol Chem. 2006 Nov 3;281(44):33450-6. doi: 10.1074/jbc.C600164200. Epub 2006 Sep 1. J Biol Chem. 2006. PMID: 16950794
-
An emerging function of survivin's BIR domain phosphorylation in the control of cell division and cell death.Cell Cycle. 2011 Mar 15;10(6):876-7. Epub 2011 Mar 15. Cell Cycle. 2011. PMID: 21350336 No abstract available.
-
Influence of casein kinase II in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human rhabdomyosarcoma cells.Clin Cancer Res. 2004 Oct 1;10(19):6650-60. doi: 10.1158/1078-0432.CCR-04-0576. Clin Cancer Res. 2004. PMID: 15475455
-
The mitotic regulator Survivin binds as a monomer to its functional interactor Borealin.J Biol Chem. 2007 Nov 30;282(48):35018-23. doi: 10.1074/jbc.M706233200. Epub 2007 Sep 19. J Biol Chem. 2007. PMID: 17881355
-
Phosphorylation of survivin at threonine 34 inhibits its mitotic function and enhances its cytoprotective activity.Cell Cycle. 2009 Jan 15;8(2):278-83. doi: 10.4161/cc.8.2.7587. Epub 2009 Jan 10. Cell Cycle. 2009. PMID: 19158485
Cited by
-
Minor Kinases with Major Roles in Cytokinesis Regulation.Cells. 2022 Nov 17;11(22):3639. doi: 10.3390/cells11223639. Cells. 2022. PMID: 36429067 Free PMC article. Review.
-
Functional Analysis of the Plant Chromosomal Passenger Complex.Plant Physiol. 2020 Aug;183(4):1586-1599. doi: 10.1104/pp.20.00344. Epub 2020 May 27. Plant Physiol. 2020. PMID: 32461300 Free PMC article.
-
Identification of a novel function of the clathrin-coated structure at the plasma membrane in facilitating GM-CSF receptor-mediated activation of JAK2.Cell Cycle. 2012 Oct 1;11(19):3611-26. doi: 10.4161/cc.21920. Epub 2012 Aug 30. Cell Cycle. 2012. PMID: 22935703 Free PMC article.
-
Overexpression of apoptosis-related protein, survivin, in fibroblasts from patients with systemic sclerosis.Ir J Med Sci. 2019 Nov;188(4):1443-1449. doi: 10.1007/s11845-019-01978-w. Epub 2019 Feb 13. Ir J Med Sci. 2019. PMID: 30761457
-
CK2 and the Hallmarks of Cancer.Biomedicines. 2022 Aug 16;10(8):1987. doi: 10.3390/biomedicines10081987. Biomedicines. 2022. PMID: 36009534 Free PMC article. Review.
References
-
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. - PubMed
-
- Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: Conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. - PubMed
-
- Sun SC, Wei L, Li M, Lin SL, Xu BZ, Liang XW, et al. Perturbation of survivin expression affects chromosome alignment and spindle checkpoint in mouse oocyte meiotic maturation. Cell Cycle. 2009;8:3365–3372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
